LEVETIRACETAM
Sun Pharmaceutical Industries Limited
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use levetiracetam injection safely and effectively. See full prescribing information for levetiracetam injection.Levetiracetam Injection for Intravenous UseInitial U.S. Approval: 1999RECENT MAJOR CHANGESIndications and Usage, Myoclonic Seizures in Patients with Juvenile Myoclonic Epilepsy (1.2) [09/2007]Dosage and Administration (2.2) [09/2007]Warnings and Precautions (5.1 , 5.3) [09/2007]INDICATIONS AND USAGELevetiracetam injection is an antiepileptic drug indicated for adjunct therapy in adults (≥16 years of age) with the following seizure types when oral administration of levetiracetam is temporarily not feasible: Partial Onset Seizures (1.1) Myoclonic Seizures in Patients with Juvenile Myoclonic Epilepsy (1.2) DOSAGE AND ADMINISTRATIONLevetiracetam injection should be diluted in 100 mL of a compatible diluent and administered intravenously as a 15-minute infusion (2.1) . Initial Exposure To Levetiracetam (2.2): Partial Onset Seizures: 1000 mg/day, given as twice-daily dosing (500 mg twice daily), increased as needed and as tolerated in increments of 1000 mg/day additional every 2 weeks to a maximum recommended daily dose of 3000 mg. Myoclonic Seizures in Patients with Juvenile Myoclonic Epilepsy: 1000 mg/day, given as twice-daily dosing (500 mg twice daily), increased by 1000 mg/day every 2 weeks to the recommended daily dose of 3000 mg. The effectiveness of doses lower than 3000 mg/day has not been adequately studied. Replacement Therapy (2.3): When switching from oral levetiracetam, the initial total daily intravenous dosage of levetiracetam should be equivalent to the total daily dosage and frequency of oral levetiracetam. At the end of the intravenous treatment period, the patient may be switched to levetiracetam oral administration at the equivalent daily dosage and frequency of the intravenous administration.See full prescribing information for dosing instructions (2.5) , adult patients with impaired renal function (2.6) , and compatibility and stability (2.7) .DOSAGE FORMS AND STRENGTHS 500 mg/5 mL single-use vial (3) CONTRAINDICATIONSNone (4) WARNINGS AND PRECAUTIONS Neuropsychiatric Adverse Reactions: Including: 1) Somnolence and fatigue, 2) Coordination difficulties and 3) Behavioral Abnormalities (e.g., psychotic symptoms, suicide ideation, and other abnormalities). (5.1) Withdrawal Seizures: Levetiracetam must be gradually withdrawn. (5.2) Side Effects Most common adverse reactions (difference in incidence rate is ≥5% between levetiracetam-treated patients and placebo-treated patients and occurred more frequently in levetiracetam-treated patients) include: somnolence, asthenia, infection, and dizziness (6.1) . Important behavioral adverse reactions (incidence of levetiracetam-treated patients > placebo-treated patients, but
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 LEVETIRACETAM INDICATIONS AND USAGE
- 2 LEVETIRACETAM DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 LEVETIRACETAM CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 LEVETIRACETAM ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 9 DRUG ABUSE AND DEPENDENCE
- 10 OVERDOSAGE
- 11 LEVETIRACETAM DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - Label - 5 mL
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - Carton - 1 x 5 mL
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Levetiracetam injection is an alternative for adult patients (16 years and older) when oral administration is temporarily not feasible.
1.1 Partial Onset Seizures
Levetiracetam injection is indicated as adjunctive therapy in the treatment of partial onset seizures in adults with epilepsy.
1.2 Myoclonic Seizures in Patients with Juvenile Myoclonic Epilepsy
Levetiracetam injection is indicated as adjunctive therapy in the treatment of myoclonic seizures in adults with juvenile myoclonic epilepsy.
2 DOSAGE AND ADMINISTRATION
2.1 General Information
Levetiracetam injection is for intravenous use only and must be diluted prior to administration. Levetiracetam injection (500 mg/5 mL) should be diluted in 100 mL of a compatible diluent [see Dosage and Administration (2.7) ] and administered intravenously as a 15-minute IV infusion.
Product with particulate matter or discoloration should not be used.
Any unused portion of the levetiracetam injection vial contents should be discarded.
2.2 Initial Exposure to Levetiracetam
Levetiracetam can be initiated with either intravenous or oral administration.
2.3 Replacement Therapy
When switching from oral levetiracetam, the initial total daily intravenous dosage of levetiracetam should be equivalent to the total daily dosage and frequency of oral levetiracetam and should be administered as a 15-minute intravenous infusion following dilution in 100 mL of a compatible diluent.
2.4 Switching to Oral Dosing
At the end of the intravenous treatment period, the patient may be switched to levetiracetam oral administration at the equivalent daily dosage and frequency of the intravenous administration.
2.5 Dosing Instructions
Levetiracetam injection is for intravenous use only and must be diluted prior to administration. One vial of levetiracetam injection contains 500 mg levetiracetam (500 mg/5 mL). See Table 1 for the recommended preparation and administration of levetiracetam injection to achieve a dose of 500 mg, 1000 mg, or 1500 mg.
| Dose | Withdraw Volume | Volume of Diluent | Infusion Time |
|---|---|---|---|
| 500 mg | 5 mL (5 mL vial) | 100 mL | 15 minutes |
| 1000 mg | 10 mL (two 5 mL vials) | 100 mL | 15 minutes |
| 1500 mg | 15 mL (three 5 mL vials) | 100 mL | 15 minutes |
For example, to prepare a 1000 mg dose, dilute 10 mL of levetiracetam injection in 100 mL of a compatible diluent [see Dosage and Administration (2.7) ] and administer intravenously as a 15-minute infusion.
2.6 Adult Patients With Impaired Renal Function
Levetiracetam dosing must be individualized according to the patient's renal function status. Recommended doses and adjustment for dose for adults are shown in Table 2. To use this dosing table, an estimate of the patient's creatinine clearance (CLcr) in mL/min is needed. CLcr in mL/min may be estimated from serum creatinine (mg/dL) determination using the following formula:
| 1 For female patients | ||
| CLcr= | [140-age (years)] x weight (kg) | x 1 0.85 |
| - - - - - - - - - - - - - - - - - - - - - - | ||
| 72 x serum creatinine (mg/dL) | ||
| Group | Creatinine Clearance (mL/min) |
Dosage (mg) |
Frequency |
| Normal | > 80 | 500 to 1,500 | Every 12 h |
| Mild | 50 – 80 | 500 to 1,000 | Every 12 h |
| Moderate | 30 – 50 | 250 to 750 | Every 12 h |
| Severe | < 30 | 250 to 500 | Every 12 h |
| ESRD patients using dialysis | ---- | 500 to 1,000 |
 |
2.7 Compatibility and Stability
Levetiracetam injection was found to be physically compatible and chemically stable when mixed with the following diluents and antiepileptic drugs for at least 24 hours and stored in polyvinyl chloride (PVC) bags at controlled room temperature 15-30°C (59-86°F).
Diluents
Sodium chloride (0.9%) injection, USP
Lactated Ringer’s injection
Dextrose 5% injection, USP
Other Antiepileptic Drugs
Lorazepam
Diazepam
Valproate sodium
There is no data to support the physical compatibility of levetiracetam injection with antiepileptic drugs that are not listed above.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
3 DOSAGE FORMS AND STRENGTHS
One vial of levetiracetam injection contains 500 mg levetiracetam (500 mg/5 mL).
4 CONTRAINDICATIONS
None.
5 WARNINGS AND PRECAUTIONS
5.1 Neuropsychiatric Side Effects
5.2 Withdrawal Seizures
Antiepileptic drugs, including levetiracetam, should be withdrawn gradually to minimize the potential of increased seizure frequency.
5.3 Hematologic Abnormalities
5.4 Hepatic Abnormalities
There were no meaningful changes in mean liver function tests (LFT) in controlled trials in adult patients; lesser LFT abnormalities were similar in drug and placebo treated patients in controlled trials (1.4%). No patients were discontinued from controlled trials for LFT abnormalities except for 1 (0.07%) adult epilepsy patient receiving open treatment.
5.5 Laboratory Tests
Although most laboratory tests are not systematically altered with levetiracetam treatment, there have been relatively infrequent abnormalities seen in hematologic parameters and liver function tests.
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The adverse reactions that result from levetiracetam injection use include all of those reported for levetiracetam tablets and oral solution. Equivalent doses of intravenous (IV) levetiracetam and oral levetiracetam result in equivalent Cmax, Cmin, and total systemic exposure to levetiracetam when the IV levetiracetam is administered as a 15 minute infusion.
The prescriber should be aware that the adverse reaction incidence figures in the following tables, obtained when levetiracetam was added to concurrent AED therapy, cannot be used to predict the frequency of adverse experiences in the course of usual medical practice where patient characteristics and other factors may differ from those prevailing during clinical studies. Similarly, the cited frequencies cannot be directly compared with figures obtained from other clinical investigations involving different treatments, uses, or investigators. An inspection of these frequencies, however, does provide the prescriber with one basis to estimate the relative contribution of drug and non-drug factors to the adverse reaction incidences in the population studied.
6.2 Postmarketing Experience
The following adverse events have been identified during postapproval use of levetiracetam. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a casual relationship to drug exposure.
In addition to the adverse reactions listed above [see Adverse Reactions (6.1) ], the following adverse events have been reported in patients receiving marketed levetiracetam worldwide. The listing is alphabetized: abnormal liver function test, hepatic failure, hepatitis, leukopenia, neutropenia, pancreatitis, pancytopenia (with bone marrow suppression identified in some of these cases), thrombocytopenia and weight loss. Alopecia has been reported with levetiracetam use; recovery was observed in majority of cases where levetiracetam was discontinued. There have been reports of suicidal behavior (including completed suicide, suicide attempt and suicidal ideation) with marketed levetiracetam. [see Patient Counseling Information (17) ].
7 DRUG INTERACTIONS
7.1 General Information
In vitro data on metabolic interactions indicate that levetiracetam is unlikely to produce, or be subject to, pharmacokinetic interactions. Levetiracetam and its major metabolite, at concentrations well above Cmax levels achieved within the therapeutic dose range, are neither inhibitors of nor high affinity substrates for human liver cytochrome P450 isoforms, epoxide hydrolase or UDP-glucuronidation enzymes. In addition, levetiracetam does not affect the in vitro glucuronidation of valproic acid.
Levetiracetam circulates largely unbound (<10% bound) to plasma proteins; clinically significant interactions with other drugs through competition for protein binding sites are therefore unlikely.
Potential pharmacokinetic interactions were assessed in clinical pharmacokinetic studies (phenytoin, valproate, oral contraceptive, digoxin, warfarin, probenecid) and through pharmacokinetic screening in the placebo-controlled clinical studies in epilepsy patients.
7.2 Phenytoin
Levetiracetam (3000 mg daily) had no effect on the pharmacokinetic disposition of phenytoin in patients with refractory epilepsy. Pharmacokinetics of levetiracetam were also not affected by phenytoin.
7.3 Valproate
Levetiracetam (1500 mg twice daily) did not alter the pharmacokinetics of valproate in healthy volunteers. Valproate 500 mg twice daily did not modify the rate or extent of levetiracetam absorption or its plasma clearance or urinary excretion. There also was no effect on exposure to and the excretion of the primary metabolite, ucb L057.
7.4 Other Antiepileptic Drugs
Potential drug interactions between levetiracetam and other AEDs (carbamazepine, gabapentin, lamotrigine, phenobarbital, phenytoin, primidone and valproate) were also assessed by evaluating the serum concentrations of levetiracetam and these AEDs during placebo-controlled clinical studies. These data indicate that levetiracetam does not influence the plasma concentration of other AEDs and that these AEDs do not influence the pharmacokinetics of levetiracetam.
7.5 Oral Contraceptives
Levetiracetam (500 mg twice daily) did not influence the pharmacokinetics of an oral contraceptive containing 0.03 mg ethinyl estradiol and 0.15 mg levonorgestrel, or of the luteinizing hormone and progesterone levels, indicating that impairment of contraceptive efficacy is unlikely. Coadministration of this oral contraceptive did not influence the pharmacokinetics of levetiracetam.
7.6 Digoxin
Levetiracetam (1000 mg twice daily) did not influence the pharmacokinetics and pharmacodynamics (ECG) of digoxin given as a 0.25 mg dose every day. Coadministration of digoxin did not influence the pharmacokinetics of levetiracetam.
7.7 Warfarin
Levetiracetam (1000 mg twice daily) did not influence the pharmacokinetics of R and S warfarin. Prothrombin time was not affected by levetiracetam. Coadministration of warfarin did not affect the pharmacokinetics of levetiracetam.
7.8 Probenecid
Probenecid, a renal tubular secretion blocking agent, administered at a dose of 500 mg four times a day, did not change the pharmacokinetics of levetiracetam 1000 mg twice daily. Css max of the metabolite, ucb L057, was approximately doubled in the presence of probenecid while the fraction of drug excreted unchanged in the urine remained the same. Renal clearance of ucb L057 in the presence of probenecid decreased 60%, probably related to competitive inhibition of tubular secretion of ucb L057. The effect of levetiracetam on probenecid was not studied.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
There are no adequate and well-controlled studies in pregnant women. In animal studies, levetiracetam produced evidence of developmental toxicity, including teratogenic effects, at doses similar to or greater than human therapeutic doses. Levetiracetam should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Administration to female rats throughout pregnancy and lactation led to increased incidences of minor fetal skeletal abnormalities and retarded offspring growth pre- and/or postnatally at doses ≥350 mg/kg/day (approximately equivalent to the maximum recommended human dose of 3000 mg [MRHD] on a mg/m2 basis) and with increased pup mortality and offspring behavioral alterations at a dose of 1800 mg/kg/day (6 times the MRHD on a mg/m2 basis). The developmental no effect dose was 70 mg/kg/day (0.2 times the MRHD on a mg/m2 basis). There was no overt maternal toxicity at the doses used in this study.
Treatment of pregnant rabbits during the period of organogenesis resulted in increased embryofetal mortality and increased incidences of minor fetal skeletal abnormalities at doses ≥600 mg/kg/day (approximately 4 times MRHD on a mg/m2 basis) and in decreased fetal weights and increased incidences of fetal malformations at a dose of 1800 mg/kg/day (12 times the MRHD on a mg/m2 basis). The developmental no effect dose was 200 mg/kg/day (1.3 times the MRHD on a mg/m2 basis). Maternal toxicity was also observed at 1800 mg/kg/day.
When pregnant rats were treated during the period of organogenesis, fetal weights were decreased and the incidence of fetal skeletal variations was increased at a dose of 3600 mg/kg/day (12 times the MRHD). 1200 mg/kg/day (4 times the MRHD) was a developmental no effect dose. There was no evidence of maternal toxicity in this study.
Treatment of rats during the last third of gestation and throughout lactation produced no adverse developmental or maternal effects at doses of up to 1800 mg/kg/day (6 times the MRHD on a mg/m2 basis).
8.2 Labor and Delivery
The effect of levetiracetam on labor and delivery in humans is unknown.
8.3 Nursing Mothers
Levetiracetam is excreted in breast milk. Because of the potential for serious adverse reactions in nursing infants from levetiracetam, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Safety and effectiveness of levetiracetam injection in patients below the age of 16 years have not been established.
8.5 Geriatric Use
Of the total number of subjects in clinical studies of levetiracetam, 347 were 65 and over. No overall differences in safety were observed between these subjects and younger subjects. There were insufficient numbers of elderly subjects in controlled trials of epilepsy to adequately assess the effectiveness of levetiracetam in these patients.
A study in 16 elderly subjects (age 61-88 years) with oral administration of single dose and multiple twice-daily doses for 10 days showed no pharmacokinetic differences related to age alone.
Levetiracetam is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
8.6 Use in Patients with Impaired Renal Function
Clearance of levetiracetam is decreased in patients with renal impairment and is correlated with creatinine clearance. Caution should be taken in dosing patients with moderate and severe renal impairment and in patients undergoing hemodialysis. The dosage should be reduced in patients with impaired renal function receiving levetiracetam and supplemental doses should be given to patients after dialysis [see Clinical Pharmacology (12.3) and Dosage and Administration (2.6) ].
9 DRUG ABUSE AND DEPENDENCE
The abuse and dependence potential of levetiracetam has not been evaluated in human studies.
10 OVERDOSAGE
Signs, Symptoms And Laboratory Findings Of Acute Overdosage In Humans
The highest known dose of oral levetiracetam received in the clinical development program was 6000 mg/day. Other than drowsiness, there were no adverse reactions in the few known cases of overdose in clinical trials. Cases of somnolence, agitation, aggression, depressed level of consciousness, respiratory depression and coma were observed with levetiracetam overdoses in postmarketing use.
Treatment Or Management Of Overdose
There is no specific antidote for overdose with levetiracetam. If indicated, elimination of unabsorbed drug should be attempted by emesis or gastric lavage; usual precautions should be observed to maintain airway. General supportive care of the patient is indicated including monitoring of vital signs and observation of the patient's clinical status. A Certified Poison Control Center should be contacted for up to date information on the management of overdose with levetiracetam.
Hemodialysis
Standard hemodialysis procedures result in significant clearance of levetiracetam (approximately 50% in 4 hours) and should be considered in cases of overdose. Although hemodialysis has not been performed in the few known cases of overdose, it may be indicated by the patient's clinical state or in patients with significant renal impairment.
11 DESCRIPTION
Levetiracetam injection is an antiepileptic drug available as a clear, colorless, sterile solution (100 mg/mL) for intravenous administration.
The chemical name of levetiracetam, a single enantiomer, is (-)-(S)-α-ethyl-2-oxo-1-pyrrolidine acetamide, its molecular formula is C8H14N2O2 and its molecular weight is 170.21. Levetiracetam is chemically unrelated to existing antiepileptic drugs (AEDs). It has the following structural formula:
Levetiracetam is a white to off-white crystalline powder with a faint odor and a bitter taste. It is very soluble in water (104 g/100 mL). It is freely soluble in chloroform (65.3 g/100 mL) and in methanol (53.6 g/100 mL), soluble in ethanol (16.5 g/100 mL), sparingly soluble in acetonitrile (5.7 g/100 mL) and practically insoluble in n-hexane. (Solubility limits are expressed as g/100 mL solvent.)
Levetiracetam injection contains 100 mg of levetiracetam per mL. It is supplied in single-use 5 mL vials containing 500 mg levetiracetam, water for injection, 45 mg sodium chloride, and buffered at approximately pH 5.5 with glacial acetic acid and 8.2 mg sodium acetate trihydrate. Levetiracetam injection must be diluted prior to intravenous infusion [see Dosage and Administration (2.1) ].
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The precise mechanism(s) by which levetiracetam exerts its antiepileptic effect is unknown. The antiepileptic activity of levetiracetam was assessed in a number of animal models of epileptic seizures. Levetiracetam did not inhibit single seizures induced by maximal stimulation with electrical current or different chemoconvulsants and showed only minimal activity in submaximal stimulation and in threshold tests. Protection was observed, however, against secondarily generalized activity from focal seizures induced by pilocarpine and kainic acid, two chemoconvulsants that induce seizures that mimic some features of human complex partial seizures with secondary generalization. Levetiracetam also displayed inhibitory properties in the kindling model in rats, another model of human complex partial seizures, both during kindling development and in the fully kindled state. The predictive value of these animal models for specific types of human epilepsy is uncertain.
In vitro and in vivo recordings of epileptiform activity from the hippocampus have shown that levetiracetam inhibits burst firing without affecting normal neuronal excitability, suggesting that levetiracetam may selectively prevent hypersynchronization of epileptiform burst firing and propagation of seizure activity.
Levetiracetam at concentrations of up to 10 μM did not demonstrate binding affinity for a variety of known receptors, such as those associated with benzodiazepines, GABA (gamma-aminobutyric acid), glycine, NMDA (N-methyl-D-aspartate), re-uptake sites, and second messenger systems. Furthermore, in vitro studies have failed to find an effect of levetiracetam on neuronal voltage-gated sodium or T-type calcium currents and levetiracetam does not appear to directly facilitate GABAergic neurotransmission. However, in vitro studies have demonstrated that levetiracetam opposes the activity of negative modulators of GABA- and glycine-gated currents and partially inhibits N-type calcium currents in neuronal cells.
A saturable and stereoselective neuronal binding site in rat brain tissue has been described for levetiracetam. Experimental data indicate that this binding site is the synaptic vesicle protein SV2A, thought to be involved in the regulation of vesicle exocytosis. Although the molecular significance of levetiracetam binding to synaptic vesicle protein SV2A is not understood, levetiracetam and related analogs showed a rank order of affinity for SV2A which correlated with the potency of their antiseizure activity in audiogenic seizure-prone mice. These findings suggest that the interaction of levetiracetam with the SV2A protein may contribute to the antiepileptic mechanism of action of the drug.
12.3 Pharmacokinetics
Equivalent doses of intravenous (IV) levetiracetam and oral levetiracetam result in equivalent Cmax, Cmin, and total systemic exposure to levetiracetam when the IV levetiracetam is administered as a 15 minute infusion.
The pharmacokinetics of levetiracetam have been studied in healthy adult subjects, adults and pediatric patients with epilepsy, elderly subjects and subjects with renal and hepatic impairment.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
ln animal studies, levetiracetam produced evidence of developmental toxicity at doses similar to or greater than human therapeutic doses.
14 CLINICAL STUDIES
All efficacy trials utilized oral formulations. The recommendation for the parenteral formulation is based upon these studies as well as the demonstration of comparable bioavailability of the oral and the parenteral formulation [see Pharmacokinetics (12.3) ].
In the following studies, statistical significance versus placebo indicates a p value <0.05.
14.1 Partial Onset Seizures
14.2 Myoclonic Seizures in Patients with Juvenile Myoclonic Epilepsy
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Levetiracetam injection 500 mg/5 mL is a clear, colorless, sterile solution. It is supplied in single-use 5 mL vials, available in cartons of 1 vial (NDC 62756-513-40) and cartons of 10 vials (NDC 62756-513-44).
16.2 Storage
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° and 30°C (59° and 86°F) [see USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
Patients should be advised to notify their physician if they are pregnant prior to therapy.
Patients should be advised that levetiracetam may cause dizziness and somnolence. Accordingly, patients should be advised not to drive or operate heavy machinery or engage in other hazardous activities until they have gained sufficient experience on levetiracetam to gauge whether it adversely affects their performance of these activities.
Patients should be advised that levetiracetam may cause changes in behavior (e.g. aggression, agitation, anger, anxiety, apathy, depression, hostility, and irritability) and in rare cases patients may experience psychotic symptoms.
Patients should be advised to immediately report any symptoms of depression and/or suicidal ideation to their prescribing physician as suicide, suicide attempt and suicidal ideation have been reported in patients treated with levetiracetam.
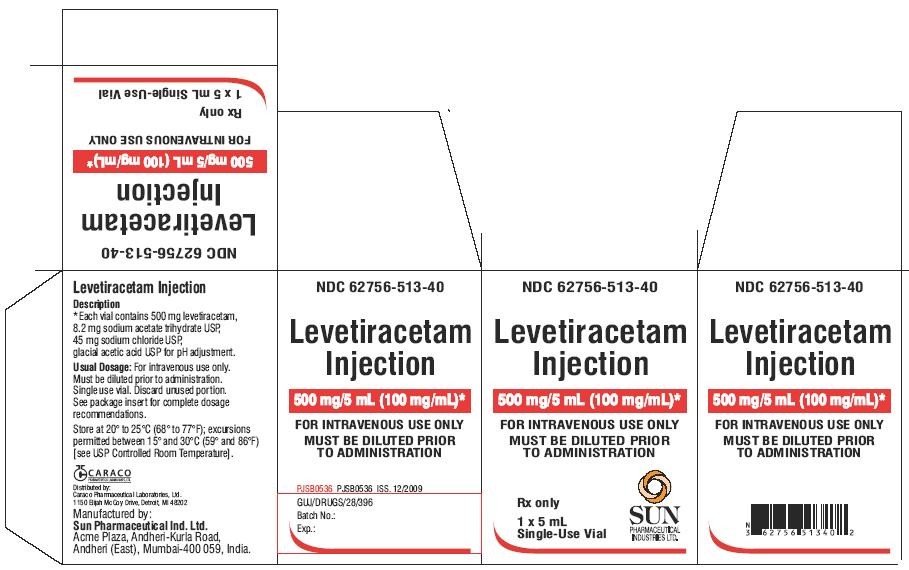
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - Label - 5 mL
NDC 62756-513-40
Levetiracetam Injection
500 mg/5 mL (100 mg/mL)
FOR INTRAVENOUS USE ONLY
Rx only
5 mL Single-Use Vial

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - Carton - 1 x 5 mL
NDC 62756-513-40
Levetiracetam Injection
500 mg/5 mL (100 mg/mL)
FOR INTRAVENOUS USE ONLY
MUST BE DILUTED PRIOR TO ADMINISTRATION
Rx only
1 x 5 mL
Single-Use Vial
SUN PHARMACEUTICAL INDUSTRIES LTD.
Study 1
Study 1 was a double-blind, placebo-controlled, parallel-group study conducted at 41 sites in the United States comparing levetiracetam 1000 mg/day (N=97), levetiracetam 3000 mg/day (N=101), and placebo (N=95) given in equally divided doses twice daily. After a prospective baseline period of 12 weeks, patients were randomized to one of the three treatment groups described above. The 18-week treatment period consisted of a 6-week titration period, followed by a 12-week fixed dose evaluation period, during which concomitant AED regimens were held constant. The primary measure of effectiveness was a between group comparison of the percent reduction in weekly partial seizure frequency relative to placebo over the entire randomized treatment period (titration + evaluation period). Secondary outcome variables included the responder rate (incidence of patients with ≥50% reduction from baseline in partial onset seizure frequency). The results of the analysis of Study 1 are displayed in Table 7.
| Placebo (N=95) |
Levetiracetam 1000 mg/day (N=97) |
Levetiracetam 3000 mg/day (N=101) |
|
| Percent reduction in partial seizure frequency over placebo | – | 26.1% |
30.1% |
The percentage of patients (y-axis) who achieved ≥50% reduction in weekly seizure rates from baseline in partial onset seizure frequency over the entire randomized treatment period (titration + evaluation period) within the three treatment groups (x-axis) is presented in Figure 1.
Figure 1: Responder Rate (≥50% Reduction from Baseline) in Study 1
* Statistically significant versus placebo
Study 2
Study 2 was a double-blind, placebo-controlled, crossover study conducted at 62 centers in Europe comparing levetiracetam 1000 mg/day (N=106), levetiracetam 2000 mg/day (N=105), and placebo (N=111) given in equally divided doses twice daily.
The first period of the study (Period A) was designed to be analyzed as a parallel-group study. After a prospective baseline period of up to 12 weeks, patients were randomized to one of the three treatment groups described above. The 16-week treatment period consisted of the 4-week titration period followed by a 12-week fixed dose evaluation period, during which concomitant AED regimens were held constant. The primary measure of effectiveness was a between group comparison of the percent reduction in weekly partial seizure frequency relative to placebo over the entire randomized treatment period (titration + evaluation period). Secondary outcome variables included the responder rate (incidence of patients with ≥50% reduction from baseline in partial onset seizure frequency). The results of the analysis of Period A are displayed in Table 8.
| Placebo (N=111) |
Levetiracetam 1000 mg/day (N=106) |
Levetiracetam 2000 mg/day (N=105) |
|
| Percent reduction in partial seizure frequency over placebo | – | 17.1% |
21.4% |
The percentage of patients (y-axis) who achieved ≥50% reduction in weekly seizure rates from baseline in partial onset seizure frequency over the entire randomized treatment period (titration + evaluation period) within the three treatment groups (x-axis) is presented in Figure 2.
Figure 2: Responder Rate (≥50% Reduction From Baseline) In Study 2: Period A
* Statistically significant versus placebo
The comparison of levetiracetam 2000 mg/day to levetiracetam 1000 mg/day for responder rate was statistically significant (P=0.02). Analysis of the trial as a cross-over yielded similar results.
Study 3
Study 3 was a double-blind, placebo-controlled, parallel-group study conducted at 47 centers in Europe comparing levetiracetam 3000 mg/day (N=180) and placebo (N=104) in patients with refractory partial onset seizures, with or without secondary generalization, receiving only one concomitant AED. Study drug was given in two divided doses. After a prospective baseline period of 12 weeks, patients were randomized to one of two treatment groups described above. The 16-week treatment period consisted of a 4-week titration period, followed by a 12-week fixed dose evaluation period, during which concomitant AED doses were held constant. The primary measure of effectiveness was a between group comparison of the percent reduction in weekly seizure frequency relative to placebo over the entire randomized treatment period (titration + evaluation period). Secondary outcome variables included the responder rate (incidence of patients with ≥50% reduction from baseline in partial onset seizure frequency). Table 9 displays the results of the analysis of Study 3.
| Placebo (N=104) |
Levetiracetam 3000 mg/day (N=180) |
|
| Percent reduction in partial seizure frequency over placebo | – | 23% |
The percentage of patients (y-axis) who achieved ≥50% reduction in weekly seizure rates from baseline in partial onset seizure frequency over the entire randomized treatment period (titration + evaluation period) within the two treatment groups (x-axis) is presented in Figure 3.
Figure 3: Responder Rate (≥50% Reduction From Baseline) In Study 3
* Statistically significant versus placebo
LEVETIRACETAMLEVETIRACETAM INJECTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||