Levocetirizine Dihydrochloride
Camber Pharmaceuticals, Inc.
Hetero Labs Limited
HIGHLIGHTS OF PRESCRIBING INFORMATION RECENT MAJOR CHANGES Warningsand Precautions, Urinary Retention (5.2) [09/2012]INDICATIONS AND USAGE11.3DOSAGE AND ADMINISTRATION2.12.22.412.3DOSAGE FORMS AND STRENGTHS3CONTRAINDICATIONS444WARNINGS AND PRECAUTIONSSide Effects6.1To report SUSPECTED ADVERSE REACTIONS, contact Hetero Labs Limited at 866-495-1995 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.USE IN SPECIFIC POPULATIONS8.612.312.3Pediatric use information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.’s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 INDICATIONS & USAGE
- 2 DOSAGE & ADMINISTRATION
- 3 DOSAGE FORMS & STRENGTHS
- 4 LEVOCETIRIZINE DIHYDROCHLORIDE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 LEVOCETIRIZINE DIHYDROCHLORIDE ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 LEVOCETIRIZINE DIHYDROCHLORIDE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
1 INDICATIONS & USAGE
1.3 Chronic Idiopathic Urticaria
Pediatric use information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.‘s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.
2 DOSAGE & ADMINISTRATION
2.1 Adults and Children 12 Years of Age and Older
2.2 Children 6 to 11 Years of Age
Clinical Pharmacology (12.3 )
2.3 Children 6 months to 5 Years of Age
Pediatric use information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.‘s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.
2.4 Dose Adjustment for Renal and Hepatic Impairment
CR
CR
CR
CR
3 DOSAGE FORMS & STRENGTHS
4 CONTRAINDICATIONS
4.1 Patients with known hypersensitivity
Patients with known hypersensitivity to levocetirizine or any of the ingredients of levocetirizine dihydrochloride tablets, or to cetirizine. Observed reactions range from urticaria to anaphylaxis [see Adverse Reactions (6.2 ) ].
4.2 Patients with end-stage renal disease
CR
4.3 Pediatric patients with impaired renal function
Pediatric use information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.‘s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.
5 WARNINGS AND PRECAUTIONS
5.1 Somnolence
5.2 Urinary Retension
Urinary retention has been reported post-marketing with levocetirizine dihydrochloride. Levocetirizine dihydrochloride should be used with caution in patients with predisposing factors of urinary retention (e.g. spinal cord lesion, prostatic hyperplasia) as levocetirizine dihydrochloride may increase the risk of urinary retention. Discontinue levocetirizine dihydrochloride if urinary retention occurs.
6 ADVERSE REACTIONS
Warnings and Precautions (5 )
6.1 Clinical Trials Experience
Adults and Adolescents 12 years of Age and Older
Table 1Adverse Reactions Reported in ≥ 2%* of Subjects Aged 12 Years and Older Exposed to Levocetirizine Dihydrochloride 2.5 mg or 5 mg Once Daily in Placebo-Controlled Clinical Trials 1 to 6 Weeks in Duration
|
Adverse
Reactions |
Levocetirizine dihydrochloride
2.5 mg (n = 421) |
Levocetirizine dihydrochloride
5 mg (n = 1070) |
Placebo
(n = 912) |
| Somnolence |
22 (5%) |
61 (6%) |
16 (2%) |
| Nasopharyngitis |
25 (6%) |
40 (4%) |
28 (3%) |
| Fatigue |
5 (1%) |
46 (4%) |
20 (2%) |
| Dry Mouth |
12 (3%) |
26 (2%) |
11 (1%) |
| Pharyngitis |
10 (2%) |
12 (1%) |
9 (1%) |
Pediatric Patients 6 to 12 Years of Age
Table 2 Adverse Reactions Reported in ≥2%* of Subjects Aged 6 to 12 Years Exposed to Levocetirizine Dihydrochloride 5 mg Once Daily in Placebo-Controlled Clinical Trials 4 and 6 Weeks in Duration
|
Adverse
Reactions |
Levocetirizine Dihydrochloride
5 mg
(n = 243) |
Placebo (n = 240)
|
| Pyrexia |
10 (4%) |
5 (2%) |
| Cough |
8 (3%) |
2 (<1%) |
| Somnolence |
7 (3%) |
1 (<1%) |
| Epistaxis |
6 (2%) |
1 (<1%) |
Clinical trial information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.‘s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.
Long-Term Clinical Trials Experience
Laboratory Test Abnormalities
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
In vitroin vivo
7.1 Antipyrine, Azithromycin, Cimetidine, Erythromycin, Ketoconazole, Theophylline, and Pseudoephedrine
7.2 Ritonavir
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B
Teratogenic Effects:
2
8.3 Nursing Mothers
2
8.4 Pediatric Use
Clinical Studies (14 )
Adverse Reactions (6.1 )
Dosage and Administration ( 2.2 ) Clinical Studies (14 ) Clinical Pharmacology (12.3)
Pediatric use information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.‘s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.
8.5 Geriatric Use
8.6 Renal Impairment
Dosage and Administration (2) Clinical Pharmacology (12.3 )
8.7 Hepatic Impairment
Clinical Pharmacology (12.3 )
10 OVERDOSAGE
22
Pediatric use information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.‘s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.
11 DESCRIPTION
1212523
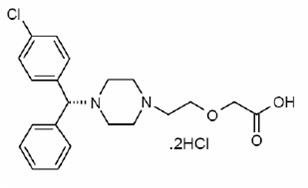
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
1In vitro1
12.2 Pharmacodynamics
12.3 Pharmacokinetics
• Absorption
maxmax
• Distribution
• Metabolism
• Elimination
Dosage and Administration (2.3 )
• Drug Interaction Studies
In vitromax
in vivo Drug Interactions (7)
• Pediatric Patients
max
Pharmacokinetic information in pediatric patients (age 1 to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.‘s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.
• Geriatric Patients
Dosage and Administration (2)
• Gender
• Race
• Renal Impairment
Dosage and Administration (2.4 )
• Hepatic Impairment
Dosage and Administration (2 )
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
2 22
Pediatricuse information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.‘s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.
13.2 Animal Pharmacology & OR Toxicology
2
2
14 CLINICAL STUDIES
14.2 Chronic Idiopathic Urticaria
Adult Patients 18 Years of Age and Older
Table 3 Mean Reflective Pruritus Severity Score in Chronic Idiopathic Urticaria Trials
|
Treatment |
N |
Baseline |
On Treatment Adjusted Mean |
Difference from Placebo |
||
|
Estimate |
95% CI
|
p-value
|
||||
|
Dose-Ranging Trial - Reflective pruritis severity score
|
||||||
|
Levocetrizine dihydrochloride 2.5 mg |
69 |
2.08 |
1.02 |
0.82 |
(0.58, 1.06)
|
<0.001
|
|
Levocetrizine dihydrochloride 5 mg |
62 |
2.07 |
0.92 |
0.91 |
(0.66, 1.16)
|
<0.001 |
|
Levocetrizine dihydrochloride 10 mg |
55 |
2.04 |
0.73 |
1.11 |
(0.85, 1.37)
|
<0.001
|
|
Placebo |
60 |
2.25 |
1.84 |
|||
|
Chronic Idiopathic Urticaria Trial - Reflective pruritis severity score |
||||||
|
Levocetrizine dihydrochloride 5 mg |
80
|
2.07
|
0.94
|
0.62
|
(0.38, 0.86)
|
<0.001
|
|
Placebo
|
82
|
2.06
|
1.56
|
|
|
|
Pediatric Patients
Use in Specific Populations (8.4 )
16 HOW SUPPLIED/STORAGE AND HANDLING
Storage:
Store at 20 to 25° C (68 to 77° F) [see USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
17.1 Somnolence
17.2 Concomitant Use of Alcohol and other Central Nervous System Depressants
Instruct patients to avoid concurrent use of levocetirizine dihydrochloride with alcohol or other central nervous system depressants because additional reduction in mental alertness may occur.
17.3 Dosing of Levocetirizine Dihydrochloride
Pediatric use information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.‘s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.

HETEROTM
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

Levocetirizine DihydrochlorideLevocetirizine Dihydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||