Levofloxacin
Northwind Pharmaceuticals
Northwind Pharmaceuticals
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
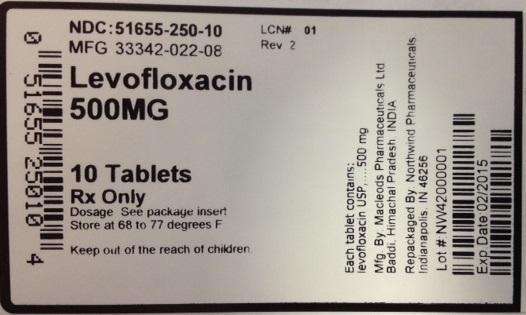
Label Display
NDC: 51655-250-10
MFG: 33342-022-08
Levofloxacin 500 mg
10 tablets
Rx only
Dosage: See package insert
Store at 68-77 degrees F.
Keep out of the reach of children.
Each tablet contains: levofloxacin USP....500 mg
Mfg. By: Macleods Pharmaceuticals Ltd Baddi, Himachai Pradesh, India
Repackaged by Northwind Pharmaceuticals, Indianapolis, IN 46256
Lot#:
Exp Date:
Warnings and Precautions
Risk of tendinitis and tendon rupture is increased. This risk is further increased in older patients usually over 60 years of age, in patients taking corticosteroids, and in patients with kidney, heart or lung transplants. Discontinue if pain or inflammation in a tendon occurs. May exacerbate muscle weakness in persons with myasthenia gravis. Avoid use in patients with a known history of myasthenia gravis. Anaphylactic reactions and allergic skin reactions, serious, occasionally fatal, may occur after first dose. Hematologic (including agranulocytosis, thrombocytopenia), and renal toxicities may occur after multiple doses. Hepatotoxicity: Severe, and sometimes fatal, hepatoxicity has been reported. Discontinue immediately if signs and symptoms of hepatitis occur. Central nervous system effects, including convulsions, anxiety, confusion, depression, and insomnia may occur after the first dose. Use with caution in patients with known or suspected disorders that may predispose them to seizures or lower the seizure threshold Increased. Intracranial pressure (pseudotumor cerebri) has been reported. Clostridium difficile-associated colitis: evaluate if diarrhea occurs. Peripheral neuropathy: discontinue immediately if symptoms occur in order to prevent irreversibility. Prolongation of the QT interval and isolated cases of torsade de pointes have been reported. Avoid use in patients with known prolongation, those with hypokalemia, and with other drugs that prolong the QT interval
Contraindications
Known hypersensitivity to levofloxacin tablets or other quinolones
Side Effects
The most common reactions (≥3%) were nausea, headache, diarrhea, insomnia, constipation and dizziness.
To report SUSPECTED ADVERSE REACTIONS, contact Macleods Pharma USA, Inc., at 1-888-943-3210 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
LevofloxacinLevofloxacin TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||