Levsin
Alaven Pharmaceutical LLC
Akorn, Inc.
Levsin® injection (hyoscyamine sulfate injection USP)
FULL PRESCRIBING INFORMATION: CONTENTS*
- LEVSIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- LEVSIN INDICATIONS AND USAGE
- LEVSIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- LEVSIN ADVERSE REACTIONS
- OVERDOSAGE
- LEVSIN DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL - 0.5 mg Carton
FULL PRESCRIBING INFORMATION
Rx Only
LEVSIN DESCRIPTION
Levsin® injection (hyoscyamine sulfate injection USP) is a sterile solution containing 0.5 mg hyoscyamine sulfate per mL in water for injection.
172332242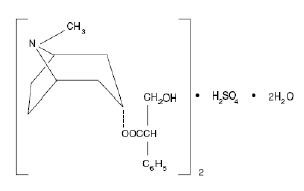
The 1 mL ampuls contain as inactive ingredients: water for injection, pH is adjusted with hydrochloric acid when necessary.
CLINICAL PHARMACOLOGY
Levsin® inhibits specifically the actions of acetylcholine on structures innervated by postganglionic cholinergic nerves and on smooth muscles that respond to acetylcholine but lack cholinergic innervation. These peripheral cholinergic receptors are present in the autonomic effector cells of the smooth muscle, cardiac muscle, the sinoatrial node, the atrioventricular node, and the exocrine glands. It is completely devoid of any action in the autonomic ganglia. Levsin® inhibits gastrointestinal propulsive motility and decreases gastric acid secretion. Levsin® also controls excessive pharyngeal, tracheal and bronchial secretions.
Levsin® disappears rapidly from the blood and is distributed throughout the entire body. The half-life of Levsin® is 3½ hours. Levsin® is partly hydrolyzed to tropic acid and tropine but the majority of the drug is excreted in the urine unchanged within the first 12 hours. Only traces of this drug are found in breast milk. Levsin® passes the blood brain barrier and the placental barrier.
LEVSIN INDICATIONS AND USAGE
Levsin® is effective as adjunctive therapy in the treatment of peptic ulcer. In acute episodes, Levsin® injection can be used to control gastric secretion, visceral spasm and hypermotility in spastic colitis, spastic bladder, cystitis, pylorospasm, and associated abdominal cramps. For use as adjunctive therapy in the treatment of irritable bowel syndrome (irritable colon, spastic colon, mucous colitis) and functional gastrointestinal disorders. Also as adjunctive therapy in the treatment of neurogenic bladder and neurogenic bowel disturbances (including the splenic flexure syndrome and neurogenic colon). Parenterally administered Levsin® is also effective in reducing gastrointestinal motility to facilitate diagnostic procedures such as endoscopy or hypotonic duodenography. Levsin® may be used to reduce pain and hypersecretion in pancreatitis, in certain cases of partial heart block associated with vagal activity, and as an antidote for poisoning by anticholinesterase agents.
-
-
-
-
LEVSIN CONTRAINDICATIONS
Glaucoma; obstructive uropathy (for example, bladder neck obstruction due to prostatic hypertrophy); obstructive disease of the gastrointestinal tract (as in achalasia, pyloroduodenal stenosis); paralytic ileus, intestinal atony of elderly or debilitated patients; unstable cardiovascular status in acute hemorrhage; severe ulcerative colitis; toxic megacolon complicating ulcerative colitis; myasthenia gravis.
WARNINGS
In the presence of high environmental temperature, heat prostration can occur with drug use (fever and heat stroke due to decreased sweating). Diarrhea may be an early symptom of incomplete intestinal obstruction, especially in patients with ileostomy or colostomy. In this instance, treatment with this drug would be inappropriate and possibly harmful. Like other anticholinergic agents, Levsin® may produce drowsiness or blurred vision. In this event, the patient should be warned not to engage in activities requiring mental alertness such as operating a motor vehicle or other machinery or to perform hazardous work while taking this drug.
Psychosis has been reported in sensitive individuals given anticholinergic drugs. CNS signs and symptoms include confusion, disorientation, short term memory loss, hallucinations, dysarthria, ataxia, coma, euphoria, decreased anxiety, fatigue, insomnia, agitation and mannerisms, and inappropriate affect. These CNS signs and symptoms usually resolve within 12-48 hours after discontinuation of the drug.
PRECAUTIONS
General
Use with caution in patients with: autonomic neuropathy, hyperthyroidism, coronary heart disease, congestive heart failure, cardiac arrhythmias, hypertension, and renal disease. Investigate any tachycardia before giving any anticholinergic drug since they may increase the heart rate. Use with caution in patients with hiatal hernia associated with reflux esophagitis.
Information for Patients
Levsin® may cause drowsiness, dizziness or blurred vision; patients should observe caution before driving, using machinery or performing other tasks requiring mental alertness.
Use of Levsin® may decrease sweating resulting in heat prostration, fever or heat stroke; febrile patients or those who may be exposed to elevated environmental temperatures should use caution.
Drug Interactions
Additive adverse effects resulting from cholinergic blockade may occur when Levsin® is administered concomitantly with other antimuscarinics, amantadine, haloperidol, phenothiazines, monoamine oxidase (MAO) inhibitors, tricyclic antidepressants or some antihistamines.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term studies in animals have been performed to determine the carcinogenic, mutagenic or impairment of fertility potential of Levsin®; however, over 30 years of marketing experience shows no demonstrable evidence of a problem.
Pregnancy
Pregnancy Category C
Animal reproduction studies have not been conducted with Levsin®. It is also not known whether Levsin® can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Levsin® should be given to a pregnant woman only if clearly needed.
Nursing Mothers
Levsin® is excreted in human milk. Caution should be exercised when Levsin® is administered to a nursing woman.
LEVSIN ADVERSE REACTIONS
Not all of the following adverse reactions have been reported with hyoscyamine sulfate. The following adverse reactions have been reported for pharmacologically similar drugs with anticholinergic/antispasmodic action. Adverse reactions may include dryness of the mouth; urinary hesitancy and retention; blurred vision; tachycardia; palpitations; mydriasis; cycloplegia; increased ocular tension; loss of taste; headache; nervousness; drowsiness; weakness; dizziness; insomnia; nausea; vomiting; impotence; suppression of lactation; constipation; bloated feeling; allergic reactions or drug idiosyncrasies; urticaria and other dermal manifestations; ataxia; speech disturbance; some degree of mental confusion and/or excitement (especially in elderly persons); and decreased sweating.
OVERDOSAGE
The signs and symptoms of overdose are headache, nausea, vomiting, blurred vision, dilated pupils, hot dry skin, dizziness, dryness of the mouth, difficulty in swallowing, and CNS stimulation.
Measures to be taken are immediate lavage of the stomach and injection of physostigmine 0.5 to 2 mg intravenously and repeated as necessary up to a total of 5 mg. Fever may be treated symptomatically (tepid water sponge baths, hypothermic blanket). Excitement to a degree which demands attention may be managed with sodium thiopental 2% solution given slowly intravenously or chloral hydrate (100-200 mL of a 2% solution) by rectal infusion. In the event of progression of the curare-like effect to paralysis of the respiratory muscles, artificial respiration should be instituted and maintained until effective respiratory action returns.
In rats, the LD50 for hyoscyamine is 375 mg/kg. Levsin® is dialyzable.
LEVSIN DOSAGE AND ADMINISTRATION
The dose may be administered subcutaneously, intramuscularly, or intravenously without dilution. As with all parenteral drug products, Levsin® injection should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Gastrointestinal Disorders
The usual adult recommended dose is 0.5 to 1 mL (0.25 to 0.5 mg). Some patients may need only a single dose; others may require administration two, three, or four times a day at four hour intervals.
Diagnostic Procedures
The usual adult recommended dose is 0.5 to 1 mL (0.25 to 0.5 mg) administered intravenously 5 to 10 minutes prior to the diagnostic procedure.
Anesthesia
Adults and pediatric patients over 2 years of age
As a pre-anesthetic medication, the recommended dose is 5 µg (0.005 mg) per kg of body weight. This dose is usually given thirty to sixty minutes prior to the anticipated time of induction of anesthesia or at the time the pre-anesthetic narcotic or sedatives are administered.
Levsin® injection may be used during surgery to reduce drug-induced bradycardia. It should be administered intravenously in increments of 0.25 mL and repeated as needed.
To achieve reversal of neuromuscular blockade, the recommended dose is 0.2 mg (0.4 mL) Levsin® injection for every 1 mg neostigmine or the equivalent dose of physostigmine or pyridostigmine.
HOW SUPPLIED
Levsin® injection (hyoscyamine sulfate injection USP, 0.5 mg/mL), is available in 1 mL ampuls.
-
Store at controlled room temperature 15°-30°C (59°-86°F).
Also available as:
| Levsin® | Dosage Strength |
Package Size |
NDC |
|---|---|---|---|
| Tablets | 0.125mg | 100 | 68220-112-10 |
| Tablets | 0.125mg | 500 | 68220-112-50 |
| Sub-lingual Tablets | 0.125mg | 100 | 68220-113-10 |
| Sub-lingual Tablets | 0.125mg | 500 | 68220-113-50 |
| Elixir | 0.125mg/5 mL | Pint | 0091-4532-16 |
| Drops | 0.125mg/mL | 15mL | 0091-4538-15 |
| Injection | 0.5mg/mL | Box of 5-1mL | 68220-111-05 |
| Levbid® extended- | 0.375mg | 100 | 68220-115-10 |
| release tablets | 0.375mg | 500 | 68220-115-50 |
Manufactured for:

Marietta, GA 30067
Address medical inquiries to:
Alaven Pharmaceutical LLC
2260 Northwest Parkway, Suite A
Marietta, GA 30067
Or call toll free 1-888-317-0001
By: Akorn Inc.
Decatur, IL 62522, USA
PC2952B
07/08
Printed in USA
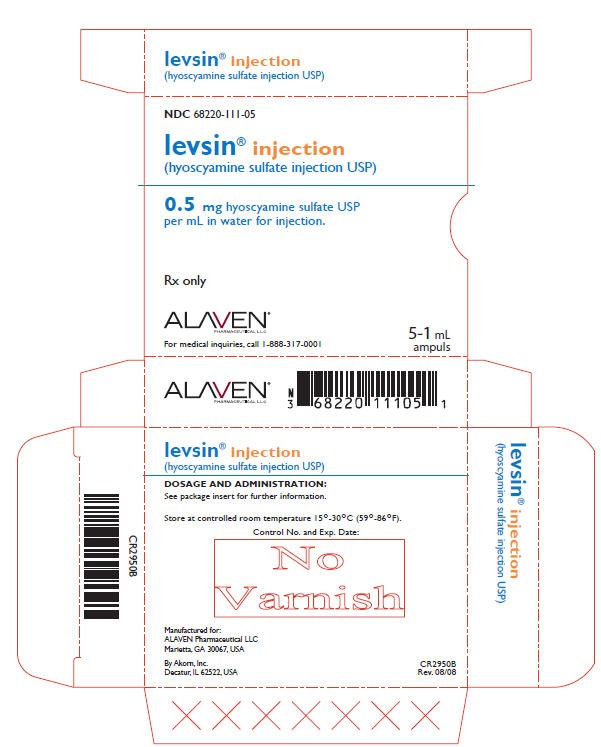
PRINCIPAL DISPLAY PANEL - 0.5 mg Carton
NDC 68220-111-05
levsin® injection
(hyoscyamine sulfate injection USP)
0.5 mg hyoscyamine sulfate USP
per mL in water for injection.
Rx only
ALAVEN®
PHARMACEUTICAL LLC
For medical inquiries, call 1-888-317-0001
5-1 mL
ampuls
LevsinHYOSCYAMINE SULFATE INJECTION, SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||