LEXXEL
LEXXEL®(enalapril maleate-felodipine ER)TABLETS
FULL PRESCRIBING INFORMATION: CONTENTS*
- USE IN PREGNANCY
- LEXXEL DESCRIPTION
- CLINICAL PHARMACOLOGY
- LEXXEL INDICATIONS AND USAGE
- LEXXEL CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- LEXXEL ADVERSE REACTIONS
- OVERDOSAGE
- LEXXEL DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
FULL PRESCRIBING INFORMATION
USE IN PREGNANCY
When used in pregnancy during the second and third trimesters, ACE inhibitors can cause injury and even death to the developing fetus. When pregnancy is detected, LEXXEL should be discontinued as soon as possible. See WARNINGS, Fetal/Neonatal Morbidity and Mortality.
LEXXEL DESCRIPTION
LEXXEL (enalapril maleate-felodipine ER) is a combination product, consisting of an outer layer of enalapril maleate surrounding a core tablet of an extended-release felodipine formulation.
Enalapril maleate is the maleate salt of enalapril, the ethyl ester of a long-acting angiotensin converting enzyme inhibitor, enalaprilat. Enalapril maleate is chemically described as (S)-1-[N-[1-(ethoxycarbonyl)-3-phenylpropyl]-L-alanyl]-L-proline, (Z)-2-butenedioate salt (1:1). Its empirical formula is C20H28N2O5•C4H4O4, and its structural formula is:
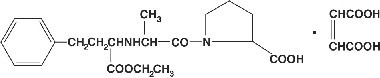
Enalapril maleate is a white to off-white, crystalline powder with a molecular weight of 492.53. It is sparingly soluble in water, soluble in ethanol, and freely soluble in methanol.
Felodipine, a calcium channel blocker, is a dihydropyridine derivative that is chemically described as ± ethyl methyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate. Its empirical formula is C18H19Cl2NO4 and its structural formula is:
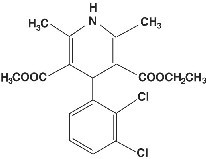
Felodipine is a slightly yellowish, crystalline powder with a molecular weight of 384.26. It is insoluble in water and is freely soluble in dichloromethane and ethanol. Felodipine is a racemic mixture; however, S-felodipine is the more biologically active enantiomer.
LEXXEL is available for oral use as tablets containing 5 mg of enalapril maleate and 5 mg of felodipine as an extended release formulation.
Inactive ingredients include: propyl gallate, polyoxyl 40 hydrogenated castor oil, cellulose compounds, lactose, aluminum silicate, sodium stearyl fumarate, carnauba wax, and black iron oxide. The tablets are imprinted with an ink of synthetic black iron oxide which contains pharmaceutical glaze in SD-45, n-butyl alcohol, propylene glycol, isopropyl alcohol, ammonium hydroxide, and methyl alcohol.
CLINICAL PHARMACOLOGY
Mechanism of Action
The two components of LEXXEL have complementary antihypertensive actions. Enalapril is a pro-drug; following oral administration, it is bioactivated by hydrolysis of the ethyl ester to enalaprilat, which is the active angiotensin converting enzyme (ACE) inhibitor. Enalaprilat inhibits angiotensin-converting enzyme in humans and animals. ACE is a peptidyl dipeptidase that catalyzes the conversion of angiotensin I to the vasoconstrictor substance, angiotensin II. Angiotensin II also stimulates aldosterone secretion by the adrenal cortex. The beneficial effects of enalapril in hypertension appear to result primarily from suppression of the renin-angiotensin-aldosterone system.
Inhibition of ACE results in decreased plasma angiotensin II, which leads to decreased vasopressor activity and to decreased aldosterone secretion. Although the latter decrease is small, it results in small increases of serum potassium. In hypertensive patients treated with enalapril maleate alone for up to 48 weeks, mean increases in serum potassium of approximately 0.2 mEq/L were observed. In patients treated with enalapril maleate plus a thiazide diuretic, there was essentially no change in serum potassium. (See PRECAUTIONS.) Removal of angiotensin II negative feedback on renin secretion leads to increased plasma renin activity.
ACE is identical to kininase, an enzyme that degrades bradykinin. Whether increased levels of bradykinin, a potent vasodepressor peptide, play a role in the therapeutic effects of enalapril maleate remains to be elucidated.
While the mechanism through which enalapril lowers blood pressure is believed to be primarily suppression of the renin-angiotensin-aldosterone system, enalapril is antihypertensive even in patients with low-renin hypertension. Although enalapril was antihypertensive in all races studied, black hypertensive patients (usually a low-renin hypertensive population) had a smaller average response to enalapril monotherapy than non-black patients.
Felodipine is a dihydropyridine calcium channel blocker that reduces the influx of Ca++ by an effect on the voltage dependent L-channels in vascular smooth muscle and cultured rabbit atrial cells, and blocks potassium-induced contracture of the rat portal vein.
Pharmacologic studies show that the effects of felodipine on contractile processes are selective, with greater effects on vascular smooth muscle than cardiac muscle. Negative inotropic effects can be detected in vitro, but such effects have not been seen in intact animals.
The consequences of vasodilation produced by felodipine include a modest, short-lived reflex increase in heart rate. A mild diuretic effect is seen in several animal species and man, but most of the effects of felodipine are accounted for by its effects on peripheral vascular resistance.
Pharmacokinetics and Metabolism
Concomitant administration of enalapril and felodipine as an extended-release formulation has little effect on the bioavailability of either compound. The rate and extent of absorption of enalapril from LEXXEL is not significantly different from that of enalapril in VASOTEC

Following oral administration of LEXXEL, peak concentrations of enalapril occur within about one hour. Enalapril is hydrolyzed to enalaprilat, which is a more potent angiotensin converting enzyme inhibitor than enalapril. Peak serum concentrations of enalaprilat occur about three hours after an oral dose of LEXXEL. Based on urinary recovery, the extent of absorption of enalapril is approximately 60%.
Peak concentrations of the isomers of felodipine are generally seen at 3-6 hours after administration of LEXXEL. Following oral administration, felodipine is almost completely absorbed and undergoes extensive first-pass metabolism; the systemic bioavailability of felodipine ER is approximately 20%.
When LEXXEL is taken with food (a substantial meal of 650 kcal or greater), some of the pharmacokinetics of its components are changed. Although the AUC(0-48 hr) of felodipine is not changed, the peak concentration of its isomers is almost doubled, and the trough concentration is approximately halved. The bioavailability of enalapril, as measured by total urinary recovery of enalaprilat, is slightly reduced. As with other dihydropyridine calcium channel blockers, the bioavailability of felodipine was increased when taken with grapefruit juice, compared to when taken with water or orange juice.
The systemic plasma clearance of felodipine in young healthy subjects is about 0.8 L/min, and the apparent volume of distribution is 10 L/kg. Approximately 99% of felodipine is bound to plasma proteins.
Following administration of 14C-labeled intravenous or immediate-release oral felodipine in man, about 70% of the dose of radioactivity was recovered in urine and 10% in the feces. A negligible amount of intact felodipine was recovered in the urine and feces (<0.5%). Six metabolites, which account for 23% of the oral dose, have been identified; none has significant vasodilating activity. Following oral administration of the immediate-release formulation, the plasma levels of felodipine declined polyexponentially with a mean terminal half-life of 11 to 16 hours.
Excretion of enalaprilat and enalapril is primarily renal. Approximately 94% of the dose is recovered in the urine and feces as enalaprilat or enalapril. The principal components in urine are enalaprilat, accounting for about 40% of the dose, and intact enalapril. There is no evidence of metabolites of enalapril, other than enalaprilat. The serum concentration profile of enalaprilat exhibits a prolonged terminal phase, apparently representing a small fraction of the administered dose that has been bound to ACE. The amount bound does not increase with dose, indicating a saturable site of binding. The effective half-life for accumulation of enalaprilat following multiple doses of enalapril maleate is 11 hours.
The disposition of enalapril and enalaprilat in patients with renal insufficiency is similar to that in patients with normal renal function until the glomerular filtration rate is reduced to 30 mL/min or less. With glomerular filtration rate ≤ 30 mL/min, peak and trough enalaprilat levels increase, time to peak concentration increases, and time to steady state may be delayed. The effective half-life of enalaprilat following multiple doses of enalapril maleate is prolonged at this level of renal insufficiency. Enalaprilat is dialyzable at a rate of 62 mL/min.
Plasma concentrations of felodipine, after a single dose and at steady state, increase with age. Mean clearance of felodipine in elderly hypertensives (mean age 74) was only 45% of that for young volunteers (mean age 26). At steady state, the mean AUC for young patients was 39% of that for the elderly. Data for intermediate age ranges suggest that the AUCs fall between the extremes of the young and the elderly.
In patients with hepatic disease, the clearance of felodipine was reduced to about 60% of that seen in normal young volunteers.
Blood Brain Barrier and Blood Placental Barrier— Animal studies have shown that felodipine crosses the blood brain barrier. The plasma to brain concentration ratio of felodipine is about 20:1. Felodipine crosses the placenta. Fetal plasma levels of felodipine are similar to maternal plasma levels. Studies in dogs indicate that enalapril crosses the blood brain barrier poorly, if at all; enalaprilat does not enter the brain. Multiple doses of enalapril maleate in rats do not result in accumulation in any tissues. Milk of lactating rats contains radioactivity following administration of 14C enalapril maleate. Radioactivity was found to cross the placenta following administration of labeled drug to pregnant hamsters.
Pharmacodynamics
Administration of enalapril maleate to patients with hypertension of severity ranging from mild to severe results in a reduction of both supine and standing blood pressure, usually with no orthostatic component. Symptomatic postural hypotension is infrequent with enalapril alone, although it might be anticipated in volume-depleted patients. (See WARNINGS.) In most patients studied, after oral administration of a single dose of enalapril, onset of antihypertensive activity was seen at one hour, with peak reduction of blood pressure achieved by 4 to 6 hours. At recommended doses, antihypertensive effects have been maintained for at least 24 hours. In some patients the effects may diminish toward the end of the dosing interval.
In most patients, achievement of optimal blood pressure reduction may require several weeks of therapy. The antihypertensive effects of enalapril have continued during long-term therapy. Abrupt withdrawal of enalapril has not been associated with a rapid increase in blood pressure. In hemodynamic studies in patients with essential hypertension, blood pressure reduction was accompanied by a reduction in peripheral arterial resistance with an increase in cardiac output and little or no change in heart rate. Following administration of enalapril maleate, there is an increase in renal blood flow; glomerular filtration rate is usually unchanged. The effects appear to be similar in patients with renovascular hypertension.
In a clinical pharmacology study, indomethacin or sulindac was administered to hypertensive patients receiving enalapril. In this study there was no evidence of a blunting of the antihypertensive action of enalapril. (See PRECAUTIONS, Drug Interactions.)
The effect of felodipine on blood pressure is principally a consequence of a dose-related decrease in peripheral vascular resistance. Blood pressure response following administration of felodipine ER to hypertensive patients is correlated with dose and plasma concentrations of felodipine. A reduction in blood pressure generally occurs within 2 to 5 hours. During chronic administration, substantial blood pressure control lasts for 24 hours, with trough reductions in diastolic blood pressure approximately 40-50% of peak reductions. A reflex increase in heart rate frequently occurs during the first week of therapy; this increase attenuates over time. Heart rate increases of 5-10 beats per minute may be seen during chronic dosing. The increase is inhibited by beta-blocking agents.
Felodipine has no significant effect on cardiac conduction (P-R, P-Q, and H-V intervals). In clinical trials in hypertensive patients without clinical evidence of left ventricular dysfunction, no symptoms suggestive of a negative inotropic effect were noted; however, none would be expected in this population.
In an 8-week, fixed-dose, parallel-group, double-blind study, 707 hypertensive patients were randomized among all possible combinations of enalapril (0, 5, or 20 mg), and extended-release felodipine (0, 2.5, 5, or 10 mg), both taken once daily. Each of the non-placebo combinations was significantly more effective than placebo in reducing seated systolic and diastolic blood pressure at peak (3 to 5 hours after dosing) and trough (24 hours after dosing). Enalapril and felodipine contributed additively to the effect, so that each active-active combination was significantly more effective than either of its component monotherapies. Most of the drug effect seen at peak was still present at trough. The efficacy of combination therapy relative to monotherapy was not significantly affected by race, sex, or age.
During chronic dosing with LEXXEL, the maximum reduction in blood pressure is generally achieved after one to two weeks. The antihypertensive effects of LEXXEL have continued during chronic therapy for at least one year.
LEXXEL INDICATIONS AND USAGE
LEXXEL is indicated for the treatment of hypertension.
This fixed combination drug is not indicated for the initial therapy of hypertension. (See DOSAGE AND ADMINISTRATION.)
In using LEXXEL, consideration should be given to the fact that another angiotensin converting enzyme inhibitor, captopril, has caused agranulocytosis, particularly in patients with renal impairment or collagen vascular disease, and that available data are insufficient to show that enalapril (a component of LEXXEL) does not have a similar risk. (See WARNINGS, Neutropenia/Agranulocytosis.)
In considering use of LEXXEL, it should be noted that black patients receiving ACE inhibitors have been reported to have a higher incidence of angioedema compared to non-blacks. (See WARNINGS, Angioedema.)
LEXXEL CONTRAINDICATIONS
LEXXEL is contraindicated in patients who are hypersensitive to any component of this product. Because of the enalapril component, LEXXEL is contraindicated in patients with a history of angioedema related to previous treatment with an angiotensin converting enzyme inhibitor and in patients with hereditary or idiopathic angioedema.
WARNINGS
Anaphylactoid and Possibly Related Reactions— Presumably because angiotensin-converting enzyme inhibitors affect the metabolism of eicosanoids and polypeptides, including endogenous bradykinin, patients receiving ACE inhibitors (including LEXXEL) may be subject to a variety of adverse reactions, some of them serious.
Angioedema: Angioedema of the face, extremities, lips, tongue, glottis, and/or larynx has been reported in patients treated with angiotensin converting enzyme inhibitors, including enalapril. This may occur at any time during treatment. In such cases LEXXEL should be promptly discontinued, and appropriate therapy and monitoring should be provided until complete and sustained resolution of signs and symptoms has occurred. In instances where swelling has been confined to the face and lips the condition has generally resolved without treatment, although antihistamines have been useful in relieving symptoms. Angioedema associated with laryngeal edema may be fatal. Where there is involvement of the tongue, glottis or larynx, likely to cause airway obstruction, appropriate therapy, e.g., subcutaneous epinephrine solution 1:1000 (0.3 mL to 0.5 mL) and/or measures necessary to ensure a patent airway, should be promptly provided. (See ADVERSE REACTIONS.)
Patients with a history of angioedema unrelated to ACE inhibitor therapy may be at increased risk of angioedema while receiving an ACE inhibitor (see also INDICATIONS AND USAGE and CONTRAINDICATIONS).
Anaphylactoid Reactions During Desensitization: Two patients undergoing desensitizing treatment with hymenoptera venom while receiving ACE inhibitors sustained life-threatening anaphylactoid reactions. In the same patients, these reactions were avoided when ACE inhibitors were temporarily withheld, but they reappeared upon inadvertent rechallenge.
Anaphylactoid Reactions During Membrane Exposure: Anaphylactoid reactions have been reported in patients dialyzed with high-flux membranes and treated concomitantly with an ACE inhibitor. Anaphylactoid reactions have also been reported in patients undergoing low-density lipoprotein apheresis with dextran sulfate absorption.
Hypotension— LEXXEL can occasionally cause symptomatic hypotension.
Excessive hypotension is rare in uncomplicated hypertensive patients treated with enalapril alone. Patients at risk for excessive hypotension, sometimes associated with oliguria and/or progressive azotemia, and rarely with acute renal failure and/or death, include those with the following conditions or characteristics: heart failure, hyponatremia, high dose diuretic therapy, recent intensive diuresis or increase in diuretic dose, renal dialysis, or severe volume and/or salt depletion of any etiology. It may be advisable to eliminate the diuretic (except in patients with heart failure), reduce the diuretic dose or increase salt intake cautiously before initiating therapy with enalapril maleate in patients at risk for excessive hypotension who are able to tolerate such adjustments. (See PRECAUTIONS, Drug Interactions and ADVERSE REACTIONS.) In patients at risk for excessive hypotension, therapy should be started under very close medical supervision and such patients should be followed closely for the first 2 weeks of treatment and whenever the dose of enalapril and/or diuretic is increased. Similar considerations may apply to patients with ischemic heart or cerebrovascular disease, in whom an excessive fall in blood pressure could result in a myocardial infarction or cerebrovascular accident.
If excessive hypotension occurs, the patient should be placed in the supine position and, if necessary, receive an intravenous infusion of normal saline. A transient hypotensive response is not a contraindication to further doses of enalapril maleate, which usually can be given without difficulty once the blood pressure has stabilized. If symptomatic hypotension develops, a dose reduction or discontinuation of enalapril or diuretic may be necessary.
Felodipine, like other calcium channel blockers, may occasionally precipitate significant hypotension and rarely syncope. It may lead to reflex tachycardia which in susceptible individuals may precipitate angina pectoris. (See ADVERSE REACTIONS.)
Neutropenia/Agranulocytosis—Another angiotensin converting enzyme inhibitor, captopril, has been shown to cause agranulocytosis and bone marrow depression, rarely in uncomplicated patients but more frequently in patients with renal impairment, especially if they also have a collagen vascular disease. Available data from clinical trials of enalapril are insufficient to show that enalapril does not cause agranulocytosis at similar rates. Marketing experience has revealed cases of neutropenia or agranulocytosis in which a causal relationship to enalapril cannot be excluded. Periodic monitoring of white blood cell counts in patients with collagen vascular disease and renal disease should be considered.
Hepatic Failure— Rarely, ACE inhibitors have been associated with a syndrome that starts with cholestatic jaundice and progresses to fulminant hepatic necrosis and (sometimes) death. The mechanism of this syndrome is not understood. Patients receiving ACE inhibitors who develop jaundice or marked elevations of hepatic enzymes should discontinue the ACE inhibitor and receive appropriate medical follow-up.
Fetal/Neonatal Morbidity and Mortality— ACE inhibitors can cause fetal and neonatal morbidity and death when administered to pregnant women. Several dozen cases have been reported in the world literature. When pregnancy is detected, LEXXEL should be discontinued as soon as possible.
The use of ACE inhibitors during the second and third trimesters of pregnancy has been associated with fetal and neonatal injury, including hypotension, neonatal skull hypoplasia, anuria, reversible or irreversible renal failure, and death. Oligohydramnios has also been reported, presumably resulting from decreased fetal renal function; oligohydramnios in this setting has been associated with fetal limb contractures, craniofacial deformation, and hypoplastic lung development. Prematurity, intrauterine growth retardation, and patent ductus arteriosus have also been reported, although it is not clear whether these occurrences were due to the ACE-inhibitor exposure.
These adverse effects do not appear to have resulted from intrauterine ACE-inhibitor exposure that has been limited to the first trimester. Mothers whose embryos and fetuses are exposed to ACE inhibitors only during the first trimester should be so informed. Nonetheless, when patients become pregnant, physicians should make every effort to discontinue the use of LEXXEL as soon as possible.
Rarely (probably less often than once in every thousand pregnancies), no alternative to ACE inhibitors will be found. In these rare cases, the mothers should be apprised of the potential hazards to their fetuses, and serial ultrasound examinations should be performed to assess the intra-amniotic environment.
If oligohydramnios is observed, LEXXEL should be discontinued unless it is considered lifesaving for the mother. Contraction stress testing (CST), a non-stress test (NST), or biophysical profiling (BPP) may be appropriate, depending upon the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury.
Infants with histories of in utero exposure to ACE inhibitors should be closely observed for hypotension, oliguria, and hyperkalemia. If oliguria occurs, attention should be directed toward support of blood pressure and renal perfusion. Exchange transfusion or dialysis may be required as means of reversing hypotension and/or substituting for disordered renal function. Enalapril, which crosses the placenta, has been removed from neonatal circulation by peritoneal dialysis with some clinical benefit, and theoretically may be removed by exchange transfusion, although there is no experience with the latter procedure.
No teratogenic effects of enalapril were seen in studies of pregnant rats and rabbits. On a body surface area basis, the doses used were 57 times and 12 times, respectively, the maximum recommended human daily dose (MRHDD).
In rats administered the combination of enalapril and felodipine (enalapril [E]=1.9-felodipine [F]=2.5 mg/kg/day), an increased incidence of fetuses with dilated renal pelvis/ureter was observed. However, there was no evidence of this effect in the offspring postweaning. In mice, with doses of E=23, F=30 mg/kg/day or greater, there was an increased incidence of both early and late in utero deaths. Other than a transient and slight decrease in body weight gain in the first generation offspring, there were no adverse effects in offspring with regard to sexual maturation, behavioral development, fertility or fecundity.
Enalapril-felodipine given to pregnant mice (enalapril 20.8, felodipine 27 mg/kg/day) and rats (enalapril =17.3, felodipine =22.5 mg/kg/day) produced plasma levels (Cmax and AUC values) of enalapril/enalaprilat that were 76 to 418-fold greater and plasma levels of felodipine that were 151 to 433-fold greater than those expected in humans (non-pregnant) at the dose to be used in humans.
PRECAUTIONS
General:
Aortic Stenosis/Hypertrophic Cardiomyopathy—As with all vasodilators, enalapril should be given with caution to patients with obstruction in the outflow tract of the left ventricle.
Impaired Renal Function— As a consequence of inhibiting the renin-angiotensin-aldosterone system, changes in renal function may be anticipated in susceptible individuals treated with enalapril. In patients with severe heart failure whose renal function may depend on the activity of the renin-angiotensin-aldosterone system, treatment with angiotensin converting enzyme inhibitors, including enalapril, may be associated with oliguria and/or progressive azotemia and rarely with acute renal failure and/or death.
In clinical studies in hypertensive patients with unilateral or bilateral renal artery stenosis, increases in blood urea nitrogen and serum creatinine were observed in 20% of patients treated with enalapril. These increases were almost always reversible upon discontinuation of enalapril and/or diuretic therapy. In such patients, renal function should be monitored during the first few weeks of therapy.
Some enalapril-treated patients with hypertension or heart failure, with no apparent pre-existing renal vascular disease, have developed increases in blood urea and serum creatinine, usually minor and transient, especially when enalapril has been given concomitantly with a diuretic. This is more likely to occur in patients with pre-existing renal impairment. Dosage reduction of enalapril or discontinuation of the diuretic may be required.
Evaluation of the hypertensive patient should always include assessment of renal function.
Hyperkalemia—Elevated serum potassium (greater than 5.7 mEq/L) was observed in approximately 1% of hypertensive patients in clinical trials treated with enalapril alone. In most cases these were isolated values which resolved despite continued therapy. Hyperkalemia was a cause of discontinuation of therapy in 0.28% of hypertensive patients. In clinical trials in heart failure, hyperkalemia was observed in 3.8% of patients but was not a cause for discontinuation.
Risk factors for the development of hyperkalemia include renal insufficiency, diabetes mellitus, and the concomitant use of potassium-sparing diuretics, potassium supplements and/or potassium-containing salt substitutes, which should be used cautiously, if at all, with enalapril. (See Drug Interactions.)
Elderly Patients or Patients with Impaired Liver Function— Patients over 65 years of age or patients with impaired liver function may have elevated plasma concentrations of felodipine. (See DOSAGE AND ADMINISTRATION.)
Cough— Presumably due to the inhibition of the degradation of endogenous bradykinin, persistent nonproductive cough has been reported with all ACE inhibitors, always resolving after discontinuation of therapy. ACE inhibitor-induced cough should be considered in the diagnosis of cough.
Surgery/Anesthesia— In patients undergoing major surgery or during anesthesia with agents that produce hypotension, enalapril may block angiotensin II formation secondary to compensatory renin release. If hypotension occurs and is considered to be due to this mechanism, it can be corrected by volume expansion.
Peripheral Edema— Peripheral edema, generally mild and not associated with generalized fluid retention, was the most common adverse event in the felodipine clinical trials. The incidence of peripheral edema was both dose and age dependent. This adverse event generally occurs within 2-3 weeks of the initiation of treatment.
Information for Patients
Patients should be instructed to take LEXXEL whole and not to divide, crush or chew the tablet.
All patients should be advised to consult their physician if they experience any of the following conditions:
Angioedema— Angioedema, including laryngeal edema, may occur at any time during treatment with angiotensin converting enzyme inhibitors, including enalapril. Patients should be so advised and told to report immediately any signs or symptoms suggesting angioedema (swelling of face, extremities, eyes, lips, tongue, difficulty in swallowing or breathing) and to take no more drug until they have consulted with the prescribing physician.
Hypotension— Patients should be cautioned to report light- headedness especially during the first few days of therapy. If actual syncope occurs, the patients should be told to discontinue LEXXEL until they have consulted with the prescribing physician. All patients should be cautioned that excessive perspiration and dehydration may lead to an excessive fall in blood pressure because of reduction in fluid volume. Other causes of volume depletion, such as vomiting or diarrhea, may also lead to a fall in blood pressure; patients should be advised to consult with the physician.
Hyperkalemia— Patients should be told not to use salt substitutes containing potassium without consulting their physician.
Neutropenia— Patients should be told to report promptly any indication of infection (eg, sore throat, fever) which may be a sign of neutropenia.
Pregnancy— Female patients of childbearing age should be told about the consequences of second- and third-trimester exposure to ACE inhibitors, and they should also be told that these consequences do not appear to have resulted from intrauterine ACE-inhibitor exposure that has been limited to the first trimester. These patients should be asked to report pregnancies to their physicians as soon as possible.
Gingival Hyperplasia— Patients should be told that mild gingival hyperplasia (gum swelling) has been reported. Good dental hygiene decreases its incidence and severity.
Note: As with many other drugs, certain advice to patients being treated with LEXXEL is warranted. This information is intended to aid in the safe and effective use of this medication. It is not a disclosure of all possible adverse or intended effects.
Drug Interactions
Hypotension — Patients on Diuretic Therapy: Patients on diuretics, and especially those in whom diuretic therapy was recently instituted, may occasionally experience an excessive reduction of blood pressure after initiation of therapy with enalapril. The possibility of hypotensive effects with enalapril can be minimized by either discontinuing the diuretic or increasing the salt intake prior to initiation of treatment with enalapril. If it is necessary to continue the diuretic, provide close medical supervision after the initial dose for at least two hours and until blood pressure has stabilized for at least an additional hour. (See WARNINGS and DOSAGE AND ADMINISTRATION.)
Agents Causing Renin Release— The antihypertensive effect of enalapril is augmented by antihypertensive agents that cause renin release (eg, diuretics).
Non-steroidal Anti-inflammatory Agents— In some patients with compromised renal function who are being treated with non-steroidal anti-inflammatory drugs, the coadministration of enalapril may result in a further deterioration of renal function. These effects are usually reversible.
In a clinical pharmacology study, indomethacin or sulindac was administered to hypertensive patients receiving VASOTEC (enalapril maleate). In this study there was no evidence of a blunting of the antihypertensive action of VASOTEC (enalapril maleate). However, reports suggest that NSAIDs may diminish the antihypertensive effect of ACE-inhibitors. This interaction should be given consideration in patients taking NSAIDs concomitantly with ACE-inhibitors.
Agents Increasing Serum Potassium— Enalapril attenuates potassium loss caused by thiazide-type diuretics. Potassium-sparing diuretics (eg, spironolactone, triamterene, or amiloride), potassium supplements, or potassium-containing salt substitutes may lead to significant increases in serum potassium. Therefore, if concomitant use of these agents is indicated because of demonstrated hypokalemia, they should be used with caution and with frequent monitoring of serum potassium.
Lithium— Lithium toxicity has been reported in patients receiving lithium concomitantly with drugs which cause elimination of sodium, including ACE inhibitors. A few cases of lithium toxicity have been reported in patients receiving concomitant enalapril and lithium and were reversible upon discontinuation of both drugs. It is recommended that serum lithium levels be monitored frequently if enalapril is administered concomitantly with lithium.
CYP3A4 Inhibitors—Felodipine is metabolized by CYP3A4. Co-administration of CYP3A4 inhibitors (eg, ketoconazole, itraconazole, erythromycin, grapefruit juice, cimetidine) with felodipine may lead to several-fold increases in the plasma levels of felodipine, either due to an increase in bioavailability or due to a decrease in metabolism. These increases in concentration may lead to increased effects, (lower blood pressure and increased heart rate). These effects have been observed with co-administration of itraconazole (a potent CYP3A4 inhibitor). Caution should be used when CYP3A4 inhibitors are co-administered with felodipine. A conservative approach to dosing felodipine should be taken. The following specific interactions have been reported:
Itraconazole—Co-administration of another extended release formulation of felodipine with itraconazole resulted in approximately 8-fold increase in the AUC, more than 6-fold increase in the Cmax, and 2-fold prolongation in the half-life of felodipine.
Erythromycin—Co-administration of felodipine (PLENDIL) with erythromycin resulted in approximately 2.5-fold increase in the AUC and Cmax, and about 2-fold prolongation in the half-life of felodipine.
Grapefruit juice—Co-administration of felodipine with grapefruit juice resulted in more than 2-fold increase in the AUC and Cmax, but no prolongation in the half-life of felodipine.
Cimetidine—Co-administration of felodipine with cimetidine (a non-specific CYP-450 inhibitor) resulted in an increase of approximately 50% in the AUC and the Cmax, of felodipine.
Beta-Blocking Agents— Enalapril has been used concomitantly with beta adrenergic-blocking agents without evidence of clinically significant adverse interactions.
A pharmacokinetic study of felodipine in conjunction with metoprolol demonstrated no significant effects on the pharmacokinetics of felodipine. The AUC and Cmax of metoprolol, however, were increased approximately 31% and 38%, respectively. In controlled clinical trials, however, beta blockers including metoprolol were concurrently administered with felodipine and were well tolerated.
Digoxin— Enalapril has been used concomitantly with digoxin without evidence of clinically significant adverse interactions.
When given concomitantly with felodipine ER, the pharmacokinetics of digoxin in patients with heart failure were not significantly altered.
Anticonvulsants— In a pharmacokinetic study, maximum plasma concentrations of felodipine were considerably lower in epileptic patients on long-term anticonvulsant therapy (eg, phenytoin, carbamazepine, or phenobarbital) than in healthy volunteers. In such patients, the mean area under the felodipine plasma concentration-time curve was also reduced to approximately 6% of that observed in healthy volunteers. Since a clinically significant interaction may be anticipated, alternative antihypertensive therapy should be considered in these patients.
Tacrolimus— Felodipine may increase the blood concentration of tacrolimus. When given concomitantly with felodipine, the tacrolimus blood concentration should be followed and the tacrolimus dose may need to be adjusted.
Other Concomitant Therapy— In healthy subjects, there were no clinically significant interactions when felodipine was given concomitantly with indomethacin or spironolactone.
Enalapril has been used concomitantly with methyldopa, nitrates, hydralazine, and prazosin without evidence of clinically significant adverse interactions.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term carcinogenicity tests have been performed with the combination. Enalapril-felodipine was not mutagenic with or without metabolic activation in vitro in the Ames microbial mutation assay, the V-79 mammalian cell forward mutation assay, the alkaline elution assay with rat hepatocytes or the CHO mammalian cell cytogenetics assay. An in vivo mouse bone marrow cytogenetics assay was also negative.
In rats given enalapril-felodipine, there was no effect on fertility in males at doses up to 6.9/9 mg/kg/day, and in females at doses up to 17.3/22.5 mg/kg/day.
There was no evidence of a tumorigenic effect when enalapril was administered for 106 weeks to male and female rats at doses up to 90 mg/kg/day or for 94 weeks to male and female mice at doses up to 90 and 180 mg/kg/day, respectively. These doses are 26 times (in rats and female mice) and 13 times (in male mice) the maximum recommended human daily dose (MRHDD) when compared on a body surface area basis.
Neither enalapril maleate nor the active diacid was mutagenic in the Ames microbial mutagen test with or without metabolic activation. Enalapril was also negative in the following genotoxicity studies: rec-assay, reverse mutation assay with E. coli, sister chromatid exchange with cultured mammalian cells, and the micronucleus test with mice, as well as in an in vivo cytogenic study using mouse bone marrow.
There were no adverse effects on reproductive performance of male and female rats treated with up to 90 mg/kg/day of enalapril (26 times the MRHDD when compared on a body surface area basis).
In a 2-year carcinogenicity study in rats fed felodipine at doses of 7.7, 23.1 or 69.3 mg/kg/day (up to 61 times

In this same rat study, a dose-related increase in the incidence of focal squamous cell hyperplasia, compared to control, was observed in the esophageal groove of male and female rats in all dose groups. No other drug-related esophageal or gastric pathology was observed in the rats or with chronic administration in mice and dogs. The latter species, like man, has no anatomical structure comparable to the esophageal groove.
Felodipine was not carcinogenic when fed to mice at doses of up to 138.6 mg/kg/day (61 times
Felodipine did not display any mutagenic activity in vitro in the Ames microbial mutagenicity test or in the mouse lymphoma forward mutation assay. No clastogenic potential was seen in vivo in the mouse micronucleus test at oral doses up to 2500 mg/kg (1100 times
A fertility study in which male and female rats were administered doses of 3.8, 9.6, or 26.9 mg/kg/day (up to 24 times
Pregnancy
Pregnancy Categories C (first trimester) and D (second and third trimesters) See WARNINGS, Fetal/Neonatal Morbidity and Mortality.
Teratogenic Effects– Studies in pregnant rabbits administered doses of felodipine 0.46, 1.2, 2.3, and 4.6 mg/kg/day (from 0.8 to 8 times
In a teratology study in cynomolgus monkeys, no reduction in the size of the terminal phalanges was observed, but an abnormal position of the distal phalanges was noted in about 40% of the fetuses.
Nonteratogenic Effects– A prolongation of parturition with difficult labor and an increased frequency of fetal and early postnatal deaths were observed in rats administered felodipine doses of 9.6 mg/kg/day (8 times
Significant enlargement of the mammary glands, in excess of the normal enlargement for pregnant rabbits, was found with doses greater than or equal to 1.2 mg/kg/day (2.1 times the maximum human dose on a mg/m2 basis). This effect occurred only in pregnant rabbits and regressed during lactation. Similar changes in the mammary glands were not observed in rats or monkeys.
There are no adequate and well-controlled studies with felodipine in pregnant women. If felodipine is used during pregnancy, or if the patient becomes pregnant while taking this drug, she should be apprised of the potential hazard to the fetus, possible digital anomalies of the infant, and the potential effects of felodipine on labor and delivery, and on the mammary glands of pregnant females.
Nursing Mothers
Enalapril and enalaprilat are detected in human breast milk. It is not known whether felodipine administered as monotherapy is secreted in human milk; studies of the combination of enalapril and felodipine in rats indicate that felodipine concentrates in milk to a level almost ten-fold that found in plasma.
Because of the potential for serious adverse reactions from enalapril and felodipine in the infant, a decision should be made either to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. Therefore, caution should be exercised when LEXXEL is given to a nursing mother.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
Clinical studies of LEXXEL did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. Elderly patients may have elevated plasma concentrations of felodipine and may respond to lower doses of felodipine. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy (see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION for information on the individual components of LEXXEL).
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection. Evaluation of the hypertensive patient should always include assessment of renal function (see CLINICAL PHARMACOLOGY).
LEXXEL ADVERSE REACTIONS
In a factorial study, combinations of enalapril at doses of 0, 5, and 20 mg and felodipine ER at doses of 0, 2.5, 5, and 10 mg were evaluated for safety in more than 700 patients with hypertension. In addition more than 500 patients received various combinations of enalapril (5 or 10 mg) and felodipine ER (2.5, 5, or 10 mg) with or without hydrochlorothiazide (12.5 mg) in an open-labeled study up to 52 weeks (mean 33 weeks). Adverse events were similar to those described with the individual components.
In general, treatment with enalapril maleate-felodipine ER was well tolerated and adverse events were mild and transient in nature. In the placebo-controlled, double-blind trial, discontinuation of therapy due to adverse events considered related (possibly, probably or definitely) occurred in 2.8% vs 1.3% of patients treated with the combination or placebo, respectively. The most frequently observed clinical adverse events considered related to treatment with the combination were headache, edema or swelling, and dizziness.
Clinical adverse events considered related (possibly, probably, or definitely) to treatment with enalapril-felodipine ER that occurred with an incidence of 1% or greater with the combination during the placebo-controlled, double-blind trial are compared to individual components and placebo in the table below:
| Body System | Enalapril | Enalapril | Felodipine | Placebo |
|---|---|---|---|---|
| Adverse Event | Felodipine | ER | ||
ER | ||||
| N=319 | N=133 | N=176 | N=79 | |
Body as a Whole | ||||
Edema/Swelling | 4.1(0.3) | 2.3 (0.0) | 10.8 (1.7) | 1.3 (0.0) |
Asthenia/Fatigue | 1.9 (0.0) | 2.3 (0.8) | 0.6 (0.6) | 3.8 (0.0) |
Nervous/Psychiatric | ||||
Headache | 10.3 (0.6) | 3.8 (0.0) | 10.2 (1.1) | 7.6 (1.3) |
Dizziness | 4.4 (0.3) | 1.5 (0.0) | 2.8 (0.6) | 0.0 (0.0) |
Respiratory | ||||
Cough | 2.2 (0.6) | 2.3 (0.0) | 0.6 (0.0) | 0.0 (0.0) |
Skin | ||||
Flushing | 1.6 (0.3) | 0.0 (0.0) | 2.3 (1.1) | 0.0 (0.0) |
Other clinical adverse events considered related (possibly, probably, or definitely) to treatment with enalapril-felodipine ER that occurred with an incidence of less than 1% in the placebo-controlled, double-blind trial are listed below. These events are listed in order of decreasing frequency within each category. Body as a Whole: Syncope, facial edema, orthostatic effects, chest pain; Cardiovascular: Palpitation, hypotension, bradycardia, premature ventricular contraction, increased blood pressure; Digestive: Dry mouth, constipation, dyspepsia, flatulence, acid regurgitation, vomiting, diarrhea, nausea, anal/rectal pain; Metabolic: Gout; Musculoskeletal: Neck pain, joint swelling; Nervous/Psychiatric: Insomnia, nervousness, somnolence, ataxia, agitation, paresthesia, tremor; Respiratory: Dyspnea, respiratory congestion, pharyngeal discomfort, dry throat; Skin: Rash, angioedema, pruritus, alopecia, dry skin; Special Senses: Increased intraocular pressure; Urogenital: Impotence, hot flashes.
Other infrequently reported adverse events were seen in clinical trials with enalapril-felodipine ER (causal relationship unknown). These included: Body as a Whole: Abdominal pain, fever; Digestive: Dental pain; Metabolic: Increased ALT and AST, hyperglycemia; Musculoskeletal: Back pain, myalgia, foot pain, knee pain, shoulder pain, tendinitis; Respiratory: Upper respiratory infection, sinusitis, pharyngitis, bronchitis, nasal congestion, influenza, sinus disorder; Special Senses: Conjunctivitis; Urogenital: Proteinuria, pyuria, urinary tract infection.
Enalapril Maleate
Other adverse events that have been reported with enalapril, without regard to causality, are listed (in decreasing severity) below:
Angioedema— Angioedema has been reported in patients receiving enalapril maleate, with an incidence higher in black than in non-black patients. Angioedema associated with laryngeal edema may be fatal. If angioedema of the face, extremities, lips, tongue, glottis and/or larynx occurs, treatment with LEXXEL should be discontinued and appropriate therapy instituted immediately. (See WARNINGS.)
Body as a Whole: Anaphylactoid reactions (see WARNINGS, Anaphylactoid and Possibly Related Reactions); Cardiovascular: Cardiac arrest, myocardial infarction or cerebrovascular accident, possibly secondary to excessive hypotension in high risk patients (see WARNINGS, Hypotension), orthostatic hypotension, pulmonary embolism and infarction, pulmonary edema, rhythm disturbances including atrial tachycardia and bradycardia, atrial fibrillation, angina pectoris; Digestive: Ileus, pancreatitis, hepatic failure, hepatitis (hepatocellular [proven on rechallenge] or cholestatic jaundice) (see WARNINGS, Hepatic Failure), melena, anorexia, glossitis, stomatitis; Hematologic: Rare cases of neutropenia, thrombocytopenia and bone marrow depression; Musculoskeletal: Muscle cramps; Nervous/Psychiatric: Depression, confusion, peripheral neuropathy (eg paresthesia, dysesthesia), vertigo; Respiratory: Bronchospasm, rhinorrhea, sore throat and hoarseness, asthma, pneumonia, pulmonary infiltrates, eosinophilic pneumonitis; Skin: Exfoliative dermatitis, toxic epidermal necrolysis, Stevens-Johnson syndrome, pemphigus, herpes zoster, erythema multiforme, urticaria, diaphoresis, photosensitivity; Special Senses: Blurred vision, taste alteration, anosmia, tinnitus, dry eyes, tearing; Urogenital: Renal failure, oliguria, renal dysfunction (see PRECAUTIONS), flank pain, gynecomastia; Miscellaneous: A symptom complex has been reported which may include a positive ANA, an elevated erythrocyte sedimentation rate, arthralgia/arthritis, myalgia/myositis, fever, serositis, vasculitis, leukocytosis, eosinophilia, photosensitivity rash and other dermatologic manifestations; Fetal/Neonatal Morbidity and Mortality: See WARNINGS, Fetal/Neonatal Morbidity and Mortality.
Felodipine as an Extended-Release Formulation
Other adverse events that have been reported with felodipine ER, without regard to causality, are listed (in decreasing severity) below:
Body as a Whole: Flu-like illness; Cardiovascular: Myocardial infarction, angina pectoris, arrhythmia, tachycardia, premature beats; Digestive: Gingival hyperplasia; Endocrine: Gynecomastia; Hematologic: Anemia; Musculoskeletal: Arthralgia, leg pain, muscle cramps, arm pain, hip pain; Nervous/Psychiatric: Depression, anxiety disorders, irritability, decreased libido; Respiratory: Upper respiratory infection, rhinorrhea, sneezing, pharyngitis, influenza, epistaxis, respiratory infection; Skin: Angioedema, contusion, erythema, urticaria, leukocytoclastic vasculitis; Special Senses: Visual disturbances; Urogenital: Urinary frequency, urinary urgency, dysuria, polyuria.
Laboratory Test Findings
In controlled clinical trials with enalapril-felodipine ER, clinically important changes in standard laboratory parameters associated with administration of LEXXEL were rare. No changes peculiar to the combination treatment were observed.
Serum Electrolytes— See PRECAUTIONS.
Creatinine— Minor reversible increases in serum creatinine were observed in patients treated with LEXXEL. Increases in creatinine are more likely to occur in patients with renal insufficiency or those pretreated with a diuretic and based on experience with other ACE inhibitors, would be expected to be especially likely in patients with renal artery stenosis (see PRECAUTIONS).
Other— Minor reversible increases or decreases in serum potassium were infrequently observed in patients treated with LEXXEL; rarely were these measurements outside the normal range.
OVERDOSAGE
Limited data are available in regard to enalapril overdosage in humans. In a suicide attempt, one patient took 150 mg felodipine together with 15 tablets each of atenolol and spironolactone and 20 tablets of nitrazepam. The patient’s blood pressure and heart rate were normal on admission to hospital; he subsequently recovered without significant sequelae.
Human overdoses with any combination of enalapril and felodipine ER have not been reported.
Single oral doses of enalapril above 1000 mg/kg and ≥1775 mg/kg were associated with lethality in mice and rats, respectively. Oral doses of felodipine at 240 mg/kg and 264 mg/kg in male and female mice, respectively, and 2390 mg/kg and 2250 mg/kg in male and female rats, respectively, caused significant lethality.
In interaction studies on the acute oral toxicity of the combination in mice, pretreatment with felodipine (50 mg/kg) for one hour led to an increase in mortality at doses of enalapril maleate that exceeded 1000 mg/kg. Significant lethality with felodipine was not increased by pretreatment of mice for one hour with 100 mg/kg of enalapril maleate.
Treatment: To obtain up-to-date information about the treatment of overdose, consult your Regional Poison-Control Center. Telephone numbers of certified poison-control centers are listed in the Physicians’ Desk Reference (PDR). In managing overdose, consider the possibilities of multiple-drug overdoses, drug-drug interactions, and unusual drug kinetics in your patient.
The most likely effect of overdose with LEXXEL is vasodilation, with consequent hypotension and tachycardia. Repletion of central fluid volume (Trendelenburg positioning, infusion of crystalloids) may be sufficient therapy, but pressor agents (norepinephrine or high-dose dopamine) may be required.
Enalaprilat may be removed from general circulation by hemodialysis at a rate of 62 mL/min and has been removed from neonatal circulation by peritoneal dialysis. (See WARNINGS, Anaphylactoid reactions during membrane exposure.) It has not been established whether felodipine can be removed from the circulation by hemodialysis.
LEXXEL DOSAGE AND ADMINISTRATION
LEXXEL is an effective treatment for hypertension. This fixed combination drug is not indicated for initial therapy of hypertension.
The recommended initial dose of enalapril maleate for hypertension in patients not receiving diuretics is 5 mg once a day. The usual dosage range of enalapril maleate for hypertension is 10-40 mg per day administered in a single dose or two divided doses. In some patients treated once daily with enalapril, the antihypertensive effect may diminish toward the end of the dosing interval. In such patients, an increase in dosage or twice daily administration should be considered. The recommended initial dose of felodipine ER is 5 mg once a day with a usual dosage range of 2.5 mg-10 mg once a day. In elderly or hepatically impaired patients, the recommended initial dose of felodipine is 2.5 mg. When LEXXEL is taken with food, the peak concentration of felodipine is almost doubled, and the trough (24-hour) concentration is approximately halved (see CLINICAL PHARMACOLOGY, Pharmacokinetics and Metabolism).
In clinical trials of enalapril-felodipine ER combination therapy using enalapril doses of 5-20 mg and felodipine ER doses of 2.5-10 mg once daily, the antihypertensive effects increased with increasing doses of each component in all patient groups.
The hazards (see WARNINGS and ADVERSE REACTIONS) of enalapril are generally independent of dose; those of felodipine are a mixture of dose-dependent phenomena (primarily peripheral edema) and dose-independent phenomena, the former much more common than the latter. Therapy with any combination of enalapril and felodipine will thus be associated with both sets of dose-independent hazards.
Rarely, the dose-independent hazards associated with enalapril or felodipine are serious. To minimize dose-independent hazards, it is usually appropriate to begin therapy with LEXXEL only after a patient has failed to achieve the desired antihypertensive effect with one or the other monotherapy.
Replacement Therapy: Although the felodipine component of LEXXEL has not been shown to be bioequivalent to the available extended-release felodipine (PLENDIL), patients receiving enalapril and felodipine from separate tablets once a day may instead wish to receive the tablets of LEXXEL containing the same component doses.
Therapy Guided By Clinical Effect: A patient whose blood pressure is not adequately controlled with felodipine (or another dihydropyridine) or enalapril (or another ACE inhibitor) alone may be switched to combination therapy with LEXXEL, initially one tablet daily of LEXXEL. If blood pressure control is inadequate after a week or two, the dose may be increased to 2 tablets administered once daily. If control remains unsatisfactory, consider addition of a thiazide diuretic.
Use in Patients with Metabolic Impairments: Regimens of therapy with LEXXEL need not be adjusted for renal function as long as the patient’s creatinine clearance is >30 mL/min/1.73m2 (serum creatinine roughly ≤3 mg/dL or 265 mmol/L). In patients with more severe renal impairment, the recommended initial dose of enalapril is 2.5 mg.
LEXXEL should regularly be taken either without food or with a light meal (see CLINICAL PHARMACOLOGY, Pharmacokinetics and Metabolism). LEXXEL should be swallowed whole and not divided, crushed or chewed.
HOW SUPPLIED
No. 3661 — Tablets LEXXEL, 5-5 are white, round/biconvex-shaped, film-coated tablets, coded LEXXEL 1, 5-5 on one side and no markings on the other. Each tablet contains 5 mg of enalapril maleate and 5 mg of felodipine as an extended-release formulation. They are supplied as follows:
NDC 0186-0001-31 unit of use bottles of 30 (with desiccants)
NDC 0186-0001-68 bottles of 100 (with desiccants)
Storage:
Store at 25°C (77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [See USP Controlled Room Temperature]. Keep container tightly closed. Protect from moisture and light. Dispense in a tight container, if product package is subdivided.
Revised 12/04
LEXXEL is a trademark of the AstraZeneca group of companies.
© AstraZeneca 2002, 2003, 2004
Manufactured for: AstraZeneca LP
Wilmington, DE 19850
By: Merck & Co., Inc., Whitehouse Station, NJ 08889, USA
9176509
620008-09
LEXXELEnalapril maleate and Felodipine TABLET, EXTENDED RELEASE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||