Lialda
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LIALDA safely and effectively. See full prescribing information for LIALDA. LIALDA (mesalamine) delayed-release tablets, for oral use Initial U.S. Approval: 1987RECENT MAJOR CHANGESWarnings and Precautions, Interference with Laboratory Tests (5.6), 08/2013INDICATIONS AND USAGELIALDA is a locally acting 5-aminosalicylic acid (5-ASA) indicated for the induction of remission in adults with active, mild to moderate ulcerative colitis and for the maintenance of remission of ulcerative colitis. (1)DOSAGE AND ADMINISTRATIONFor induction of remission of active, mild to moderate ulcerative colitis, two to four 1.2 g tablets taken once daily with food. (1, 2)For maintenance of remission of ulcerative colitis, two 1.2 g tablets taken once daily with food. (1, 2)DOSAGE FORMS AND STRENGTHSDelayed-Release Tablets: 1.2 g (3)CONTRAINDICATIONSPatients with known hypersensitivity to salicylates or aminosalicylates or to any of the ingredients of LIALDA tablets. (4)WARNINGS AND PRECAUTIONS Renal impairment may occur. Assess renal function at the beginning of treatment and periodically during treatment. (5.1) Mesalamine-induced acute intolerance syndrome has been reported. Observe patients closely for worsening of these symptoms while on treatment. (5.2) Use caution when treating patients who are hypersensitive to sulfasalazine. (5.3) Mesalamine-induced cardiac hypersensitivity reactions (myocarditis and pericarditis) have been reported. (5.3) Hepatic failure has been reported in patients with pre-existing liver disease. Use caution when treating patients with liver disease. (5.4) Upper GI tract obstruction may delay onset of action. (5.5) Use of mesalamine may lead to spuriously elevated test results when measuring urinary normetanephrine by liquid chromatography with electrochemical detection. (5.6) Side Effects The most common adverse reactions (incidence ≥ 2%) are ulcerative colitis, headache, flatulence, liver function test abnormality, and abdominal pain. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Shire US Inc. at 1-800-828-2088 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch DRUG INTERACTIONS Nephrotoxic agents including NSAIDs: renal reactions have been reported. (7.1) Azathioprine or 6-mercaptopurine: blood disorders have been reported. (7.2) USE IN SPECIFIC POPULATIONS Renal impairment: Use LIALDA with caution in patients with a history of renal disease. (5.1, 7.1, 8.5, 13.2) Nursing Women: Caution should be exercised when administered to a nursing woman. (8.3) Geriatric Patients: Monitor blood cell counts in geriatric patients. (8.5)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 LIALDA INDICATIONS AND USAGE
- 2 LIALDA DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 LIALDA CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 LIALDA ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 LIALDA DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- NDC 54092-476-12 - 1.2g 120 Count Bottle
- NDC 54092-476-08 Display Carton and Sample Carton with Blister Pack
- NDC 54092-476-01 - 1.2g 120 Count Patient Sample Bottle
- NDC 54092-476-02 - 1.2g Carton of 12 bottles containing 120 tablets each
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
LIALDA is indicated for the induction of remission in patients with active, mild to moderate ulcerative colitis and for the maintenance of remission of ulcerative colitis.
2 DOSAGE AND ADMINISTRATION
The recommended dosage for the induction of remission in adult patients with active, mild to moderate ulcerative colitis is two to four 1.2 g tablets taken once daily with a meal for a total daily dose of 2.4 g or 4.8 g. The recommended dosage for the maintenance of remission is two 1.2 g tablets taken once daily with a meal for a total daily dose of 2.4 g.
3 DOSAGE FORMS AND STRENGTHS
The red-brown ellipsoidal delayed-release tablet containing 1.2 g mesalamine is debossed on one side and imprinted with S476.
4 CONTRAINDICATIONS
LIALDA is contraindicated in patients with known hypersensitivity to salicylates or aminosalicylates or to any of the ingredients of LIALDA [see Warnings and Precautions (5.3) , Description (11) , Adverse Reactions (6.2) ].
5 WARNINGS AND PRECAUTIONS
5.1 Renal Impairment
Renal impairment, including minimal change nephropathy, acute and chronic interstitial nephritis, and, rarely, renal failure, has been reported in patients given products such as LIALDA that contain mesalamine or are converted to mesalamine.
It is recommended that patients have an evaluation of renal function prior to initiation of LIALDA therapy and periodically while on therapy. Exercise caution when using LIALDA in patients with known renal dysfunction or a history of renal disease.
In animal studies, the kidney was the principal organ for toxicity. [See Drug Interactions (7.1) and Nonclinical Toxicology (13.2)]
5.2 Mesalamine-Induced Acute Intolerance Syndrome
Mesalamine has been associated with an acute intolerance syndrome that may be difficult to distinguish from an exacerbation of ulcerative colitis. Although the exact frequency of occurrence has not been determined, it has occurred in 3% of patients in controlled clinical trials of mesalamine or sulfasalazine. Symptoms include cramping, acute abdominal pain and bloody diarrhea, and sometimes fever, headache, and rash. Observe patients closely for worsening of these symptoms while on treatment. If acute intolerance syndrome is suspected, promptly discontinue treatment with LIALDA.
5.3 Hypersensitivity Reactions
Some patients who have experienced a hypersensitivity reaction to sulfasalazine may have a similar reaction to LIALDA tablets or to other compounds that contain or are converted to mesalamine.
Mesalamine-induced cardiac hypersensitivity reactions (myocarditis and pericarditis) have been reported with LIALDA and other mesalamine medications. Caution should be taken in prescribing this medicine to patients with conditions predisposing them to the development of myocarditis or pericarditis.
5.4 Hepatic Impairment
There have been reports of hepatic failure in patients with pre-existing liver disease who have been administered mesalamine. Caution should be exercised when administering LIALDA to patients with liver disease.
5.5 Upper GI Tract Obstruction
Pyloric stenosis or other organic or functional obstruction in the upper gastrointestinal tract may cause prolonged gastric retention of LIALDA which would delay mesalamine release in the colon.
5.6 Interference with Laboratory Tests
Use of mesalamine may lead to spuriously elevated test results when measuring urinary normetanephrine by liquid chromatography with electrochemical detection because of the similarity in the chromatograms of normetanephrine and mesalamine's main metabolite, N-acetylaminosalicylic acid (N-Ac-5-ASA). An alternative, selective assay for normetanephrine should be considered.
6 ADVERSE REACTIONS
The most serious adverse reactions seen in Lialda clinical trials or with other products that contain or are metabolized to mesalamine are:
- Renal impairment, including renal failure [See Warnings and Precautions (5.1)]
- Mesalamine-induced acute intolerance syndrome [See Warnings and Precautions (5.2)]
- Hypersensitivity reactions [See Warnings and Precautions (5.3)]
- Hepatic impairment, including hepatic failure [See Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
LIALDA has been evaluated in 1368 ulcerative colitis patients in controlled and open-label trials.
Induction of Remission
In two 8-week placebo-controlled clinical trials involving 535 ulcerative colitis patients, 356 received 2.4 g/day or 4.8 g/day LIALDA tablets and 179 received placebo. The most frequent adverse reaction leading to discontinuation from LIALDA therapy was exacerbation of ulcerative colitis (0.8%). Pancreatitis occurred in less than 1% of patients during clinical trials and resulted in discontinuation of therapy with LIALDA in patients experiencing this event.
Adverse reactions occurring in LIALDA or placebo groups at a frequency of at least 1% in two 8-week, double blind, placebo-controlled trials are listed in Table 1. The most common adverse reactions with LIALDA 2.4 g/day and 4.8 g/day were headache (5.6% and 3.4%, respectively) and flatulence (4% and 2.8%, respectively).
Table 1: Adverse Reactions in Two Eight-Week Placebo-Controlled Trials Experienced by at Least 1% of the LIALDA Group and at a Rate Greater than Placeboa
|
Adverse Reaction |
LIALDA 2.4 g/day (n = 177) |
LIALDA 4.8 g/day (n = 179) |
Placebo (n = 179) |
| Headache | 10 (5.6%) | 6 (3.4%) | 1 (0.6%) |
| Flatulence | 7 (4%) | 5 (2.8%) | 5 (2.8%) |
| Liver Function Test Abnormal | 1 (0.6%) | 4 (2.2%) | 2 (1.1%) |
| Alopecia | 0 | 2 (1.1%) | 0 |
| Pruritus | 1 (0.6%) | 2 (1.1%) | 2 (1.1%) |
| a: Adverse reactions for which the placebo rate equalled or exceeded the rate for at least one of the LIALDA treatment groups were abdominal pain, dizziness, dyspepsia, and nausea. | |||
The following adverse reactions, presented by body system, were reported infrequently (less than 1%) by LIALDA-treated ulcerative colitis patients in the two controlled trials.
Cardiac Disorder: tachycardia
Vascular Disorders: hypertension, hypotension
Skin and Subcutaneous Tissue Disorders: acne, prurigo, rash, urticaria
Gastrointestinal Disorders: abdominal distention, colitis, diarrhea, pancreatitis, rectal polyp, vomiting
Investigations: decreased platelet count
Musculoskeletal and Connective Tissue Disorders: arthralgia, back pain
Nervous System Disorders: somnolence, tremor
Respiratory, Thoracic and Mediastinal Disorders: pharyngolaryngeal pain
General Disorders and Administrative Site Disorders: asthenia, face edema, fatigue, pyrexia
Ear and Labyrinth Disorders: ear pain
Maintenance of Remission of Ulcerative Colitis
The dose evaluated in three studies of LIALDA given for the maintenance of remission in patients with ulcerative colitis was 1.2 g twice daily or 2.4 g/once daily. One of these studies was a 6-month double-blind comparator study while two were 12- to 14-month open-label studies.
The most common adverse reactions with LIALDA in the maintenance arms of long-term trials were colitis ulcerative (5.8%), headache (2.9%), liver function test abnormal (2.3%), and abdominal pain (2.2%). Of the 1082 subjects in the all maintenance studies pooled, 1.9% had severe adverse reactions. The most common severe adverse reactions were gastrointestinal disorders; these were mainly symptoms associated with ulcerative colitis.
Table 2: Adverse Reactions in Three Maintenance Trials Experienced by at Least 1% of the LIALDA Group (maintenance phases of trials)
| All LIALDA (n=1082) |
||
| Adverse Reaction |
n | % |
| Colitis ulcerative | 63 | (5.8%) |
| Headache | 31 | (2.9%) |
| Liver function test abnormal | 25 | (2.3%) |
| Abdominal pain | 24 | (2.2%) |
| Diarrhea | 18 | (1.7%) |
| Abdominal distension | 14 | (1.3%) |
| Abdominal pain upper | 13 | (1.2%) |
| Dyspepsia | 13 | (1.2%) |
| Back pain | 13 | (1.2%) |
| Rash | 13 | (1.2%) |
| Arthralgia | 12 | (1.1%) |
| Fatigue | 11 | (1.0%) |
| Hypertension | 10 | (1.0%) |
The following adverse reactions, presented by body system, were reported infrequently (less than 1%) by LIALDA-treated ulcerative colitis patients in the three long-term maintenance trials (maintenance phases of these trials):
Cardiac Disorder: tachycardia
Skin and Subcutaneous Tissue Disorders: acne, alopecia, pruritis, urticaria
Gastrointestinal Disorders: colitis, flatulence, nausea, pancreatitis, rectal polyp, vomiting
Nervous System Disorders: dizziness
Respiratory, Thoracic and Mediastinal Disorders: pharyngolaryngeal pain
General Disorders and Administrative Site Disorders: asthenia, pyrexia
Ear and Labyrinth Disorders: ear pain
6.2 Postmarketing Experience
In addition to the adverse reactions reported above in clinical trials involving LIALDA, the adverse reactions listed below have been identified during post-approval use of LIALDA and other mesalamine-containing products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a Whole: lupus-like syndrome, drug fever
Cardiac Disorders: pericarditis, pericardial effusion, myocarditis
Gastrointestinal: pancreatitis, cholecystitis, gastritis, gastroenteritis, gastrointestinal bleeding, perforated peptic ulcer
Hepatic: jaundice, cholestatic jaundice, hepatitis, liver necrosis, liver failure, Kawasaki-like syndrome including changes in liver enzymes
Hematologic: agranulocytosis, aplastic anemia
Immune System Disorders: anaphylactic reaction, angioedema, Stevens-Johnson syndrome (SJS), drug reaction with eosinophilia and systemic symptoms (DRESS)
Musculoskeletal and Connective Tissue Disorders: myalgia
Neurological/Psychiatric: peripheral neuropathy, Guillain-Barre syndrome, transverse myelitis
Renal Disorders: interstitial nephritis
Respiratory, Thoracic and Mediastinal Disorders: hypersensitivity pneumonitis (including interstitial pneumonitis, allergic alveolitis, eosinophilic pneumonitis)
Skin: psoriasis, pyoderma gangrenosum, erythema nodosum
Urogenital: reversible oligospermia
7 DRUG INTERACTIONS
No investigations of interaction between LIALDA and other drugs except for certain antibiotics have been performed [see Pharmacokinetics (12.3)]. However, the following drug-drug interactions have been reported for products containing mesalamine:
7.1 Nephrotoxic Agents, Including Non-Steroidal Anti-Inflammatory Drugs
The concurrent use of mesalamine with known nephrotoxic agents, including non-steroidal anti-inflammatory drugs (NSAIDs) may increase the risk of renal reactions.
7.2 Azathioprine or 6-mercaptopurine
The concurrent use of mesalamine with azathioprine or 6-mercaptopurine may increase the risk for blood disorders.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B. Reproduction studies with mesalamine have been performed in rats at doses up to 1000 mg/kg/day (1.8 times the maximum recommended human dose based on a body surface area comparison) and rabbits at doses up to 800 mg/kg/day (2.9 times the maximum recommended human dose based on a body surface area comparison) and have revealed no evidence of impaired fertility or harm to the fetus due to mesalamine. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Mesalamine is known to cross the placental barrier.
8.3 Nursing Mothers
Low concentrations of mesalamine and higher concentrations of its N-acetyl metabolite have been detected in human breast milk. The clinical significance of this has not been determined and there is limited experience of nursing women using mesalamine. Caution should be exercised if LIALDA is administered to a nursing woman.
8.4 Pediatric Use
Safety and effectiveness of LIALDA in pediatric patients have not been established.
8.5 Geriatric Use
Reports from uncontrolled clinical studies and postmarketing reporting systems suggested a higher incidence of blood dyscrasias, i.e., neutropenia and pancytopenia in patients who were 65 years or older who were taking mesalamine-containing products such as LIALDA. Caution should be taken to closely monitor blood cell counts during mesalamine therapy.
Clinical trials of LIALDA did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. Systemic exposures are increased in elderly subjects. [see Clinical Pharmacology (12.3)]. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concurrent disease or other drug therapy in elderly patients.
10 OVERDOSAGE
LIALDA is an aminosalicylate, and symptoms of salicylate toxicity may include tinnitus, vertigo, headache, confusion, drowsiness, sweating, seizures, hyperventilation, dyspnea, vomiting, and diarrhea. Severe intoxication may lead to disruption of electrolyte balance and blood-pH, hyperthermia, dehydration, and end organ damage.
There is no specific known antidote for mesalamine overdose; however, conventional therapy for salicylate toxicity may be beneficial in the event of acute overdosage. Fluid and electrolyte imbalance should be corrected by the administration of appropriate intravenous therapy. Adequate renal function should be maintained.
11 DESCRIPTION
Each LIALDA delayed-release tablet for oral administration contains 1.2 g 5-aminosalicylic acid (5-ASA; mesalamine), an anti-inflammatory agent. Mesalamine also has the chemical name 5-amino-2-hydroxybenzoic acid and its structural formula is:
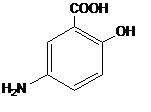
Molecular formula: C7H7NO3
Molecular weight: 153.14
The tablet is coated with a pH dependent polymer film, which breaks down at or above pH 6.8, normally in the terminal ileum where mesalamine then begins to be released from the tablet core. The tablet core contains mesalamine with hydrophilic and lipophilic excipients and provides for extended release of mesalamine.
The inactive ingredients of LIALDA are sodium carboxymethylcellulose, carnauba wax, stearic acid, silica (colloidal hydrated), sodium starch glycolate (type A), talc, magnesium stearate, methacrylic acid copolymer types A and B, triethylcitrate, titanium dioxide, red ferric oxide and polyethylene glycol 6000.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of mesalamine is not fully understood, but appears to have a topical anti-inflammatory effect on the colonic epithelial cells. Mucosal production of arachidonic acid metabolites, both through the cyclooxygenase and lipoxygenase pathways, is increased in patients with chronic inflammatory bowel disease, and it is possible that mesalamine diminishes inflammation by blocking cyclooxygenase and inhibiting prostaglandin production in the colon.
Mesalamine has the potential to inhibit the activation of nuclear factor kappa B (NFκB) and consequently the production of key pro-inflammatory cytokines. It has been proposed that reduced expression of PPARγ nuclear receptors (γ-form of peroxisome proliferator-activated receptors) may be implicated in ulcerative colitis. There is evidence that mesalamine produces pharmacodynamic effects through direct activation of PPARγ receptors in the colonic/rectal epithelium.
12.2 Pharmacodynamics
The pharmacodynamic actions of mesalamine occur in the colonic/rectal mucosae local to the delivery of drug from LIALDA into the lumen. There is information suggesting that severity of colonic inflammation in ulcerative colitis patients treated with mesalamine is inversely correlated with mucosal concentrations of mesalamine. Plasma concentrations representing systemically absorbed mesalamine are not believed to contribute extensively to efficacy.
12.3 Pharmacokinetics
Absorption
The total absorption of mesalamine from LIALDA 2.4 g or 4.8 g given once daily for 14 days to healthy volunteers was found to be approximately 21-22% of the administered dose.
Gamma-scintigraphy studies have shown that a single dose of LIALDA 1.2 g (one tablet) passed intact through the upper gastrointestinal tract of fasted healthy volunteers. Scintigraphic images showed a trail of radio-labeled tracer in the colon, suggesting that mesalamine had distributed through this region of the gastrointestinal tract.
In a single dose study, LIALDA 1.2 g, 2.4 g and 4.8 g were administered in the fasted state to healthy subjects. Plasma concentrations of mesalamine were detectable after 2 hours and reached a maximum by 9-12 hours on average for the doses studied. The pharmacokinetic parameters are highly variable among subjects (Table 3). Mesalamine systemic exposure in terms of area under the plasma concentration-time curve (AUC) was slightly more than dose proportional between 1.2 g and 4.8 g LIALDA. Maximum plasma concentrations (Cmax) of mesalamine increased approximately dose proportionately between 1.2 g and 2.4 g and sub-proportionately between 2.4 g and 4.8 g LIALDA, with the dose normalized value at 4.8 g representing, on average, 74% of that at 2.4 g based on geometric means.
Table 3: Mean (SD) PK Parameters for Mesalamine Following Single Dose Administration of LIALDA Under Fasting Conditions
| Parameter1 of Mesalamine |
LIALDA 1.2 g (N=47) |
LIALDA 2.4 g (N=48) |
LIALDA 4.8 g (N=48) |
| AUC0-t (ng.h/mL) | 9039+ (5054) | 20538 (12980) | 41434 (26640) |
| AUC0-∞ (ng.h/mL) | 9578• (5214) | 21084 (13185) | 44775# (30302) |
| Cmax (ng/mL) | 857 (638) | 1595 (1484) | 2154 (1140) |
| Tmax* (h) | 9.0** (4.0-32.1) | 12.0 (4.0-34.1) | 12.0 (4.0-34.0) |
| Tlag* (h) | 2.0** (0-8.0) | 2.0 (1.0-4.0) | 2.0 (1.0-4.0) |
| T1/2 (h) (Terminal Phase) | 8.56• (6.38) | 7.05§ (5.54) | 7.25# (8.32) |
| 1 Arithmetic mean of parameter values are presented except for Tmax and Tlag. *Median (min, max); +N=43, •N=27, §N=33, #N=36, **N=46 |
|||
Administration of a single dose of LIALDA 4.8 g with a high fat meal resulted in further delay in absorption, and plasma concentrations of mesalamine were detectable 4 hours following dosing. However, a high fat meal increased systemic exposure of mesalamine (mean Cmax: ↑ 91%; mean AUC: ↑ 16%) compared to results in the fasted state. LIALDA was administered with food in the controlled clinical trials that supported its approval.
In a single and multiple dose pharmacokinetic study of LIALDA, 2.4 g or 4.8 g was administered once daily with standard meals to 28 healthy volunteers per dose group. Plasma concentrations of mesalamine were detectable after 4 hours and were maximal by 8 hours after the single dose. Steady state was achieved generally by 2 days after dosing. Mean AUC at steady state was only modestly greater (1.1- to 1.4-fold) than predictable from single dose pharmacokinetics.
In a single dose pharmacokinetic study of LIALDA, 4.8 g was administered in the fasted state to 71 healthy male and female volunteers (28 young (18-35yrs); 28 elderly (65-75yrs); 15 elderly (>75yrs)). Increased age resulted in increased systemic exposure (approximately 2-fold in Cmax), to mesalamine and its metabolite N-acetyl-5-aminosalicylic acid. Increased age resulted in a slower apparent elimination of mesalamine, though there was high between-subject variability. Systemic exposures in individual subjects were inversely correlated with renal function as assessed by estimated creatinine clearance.
Table 4: Mean (SD) PK Parameters for Mesalamine Following Single Dose Administration of LIALDA 4.8 g under Fasting Conditions to Young and Elderly Subjects
| Parameter of 5-ASA |
Young Subjects (18-35 yrs) (N=28) |
Elderly Subjects (65-75 yrs) (N=28) |
Elderly Subjects (>75 yrs) (N=15) |
| AUC0-t (ng.h/mL) | 51570 (23870) | 73001 (42608) | 65820 (25283) |
| AUC0-∞ (ng.h/mL) | 58057b (22429) | 89612c (40596) | 63067d (22531) |
| Cmax (ng/mL) | 2243 (1410) | 4999 (4381) | 4832 (4383) |
| tmax a (h) | 22.0 (5.98 - 48.0) | 12.5 (4.00 - 36.0) | 16.0 (4.00 - 26.0) |
| tlag a (h) | 2.00 (1.00 - 6.00) | 2.00 (1.00 - 4.00) | 2.00 (2.00 - 4.00) |
| t1/2 (h), terminal phase | 5.68b (2.83) | 9.68c (7.47) | 8.67d (5.84) |
| Renal clearance (L/h) | 2.05 (1.33) | 2.04 (1.16) | 2.13 (1.20) |
| Arithmetic mean (SD) data are presented, N = Number of subjects a Median (min-max), bN=15, cN=16, dN=13 |
|||
Distribution
Mesalamine is approximately 43% bound to plasma proteins at the concentration of 2.5 μg/mL.
Metabolism
The only major metabolite of mesalamine (5-aminosalicylic acid) is N-acetyl-5-aminosalicylic acid. Its formation is brought about by N-acetyltransferase (NAT) activity in the liver and intestinal mucosa cells, principally by NAT-1.
Elimination
Elimination of mesalamine is mainly via the renal route following metabolism to N-acetyl-5-aminosalicylic acid (acetylation). However, there is also limited excretion of the parent drug in urine. Of the approximately 21-22% of the dose absorbed, less than 8% of the dose was excreted unchanged in the urine after 24 hours, compared with greater than 13% for N-acetyl-5-aminosalicylic acid. The apparent terminal half-lives for mesalamine and its major metabolite after administration of LIALDA 2.4 g and 4.8 g were, on average, 7-9 hours and 8-12 hours, respectively.
Drug Interactions:
The potential effect of Lialda (4.8 g given once daily) on the pharmacokinetics of four commonly used antibiotics were evaluated in healthy subjects. The four antibiotics studied and their dosing regimens were as follows: amoxicillin (single 500 mg dose), ciprofloxacin XR (single 500 mg dose), metronidazole (750 mg twice daily for 3.5 days), and sulfamethoxazole/trimethoprim (800 mg/160 mg twice daily for 3.5 days). Coadministration of Lialda did not result in clinically significant changes in the pharmacokinetics of any of the four antibiotics. The change in Cmax and AUC of amoxicillin, ciprofloxacin and metronidazole when they were co-administered with Lialda were all ≤ 3%. There was an increase of 12% in Cmax and an increase of 15% in AUC of sulfamethoxazole when sulfamethoxazole/trimethoprim was coadministered with Lialda [see Drug Interactions (7)].
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a 104-week dietary carcinogenicity study in CD-1 mice, mesalamine at doses up to 2500 mg/kg/day was not tumorigenic. This dose is 2.2 times the maximum recommended human dose (based on a body surface area comparison) of LIALDA. Furthermore, in a 104-week dietary carcinogenicity study in Wistar rats, mesalamine up to a dose of 800 mg/kg/day was not tumorigenic. This dose is 1.4 times the recommended human dose (based on a body surface area comparison) of LIALDA.
Mutagenesis
No evidence of mutagenicity was observed in an in vitro Ames test or an in vivo mouse micronucleus test.
Impairment of Fertility
No effects on fertility or reproductive performance were observed in male or female rats at oral doses of mesalamine up to 400 mg/kg/day (0.7 times the maximum recommended human dose based on a body surface area comparison).
13.2 Animal Toxicology and/or Pharmacology
In animal studies with mesalamine, a 13-week oral toxicity study in mice and 13-week and 52-week oral toxicity studies in rats and cynomolgus monkeys have shown the kidney to be the major target organ of mesalamine toxicity. Oral daily doses of 2400 mg/kg in mice and 1150 mg/kg in rats produced renal lesions including granular and hyaline casts, tubular degeneration, tubular dilation, renal infarct, papillary necrosis, tubular necrosis, and interstitial nephritis. In cynomolgus monkeys, oral daily doses of 250 mg/kg or higher produced nephrosis, papillary edema, and interstitial fibrosis.
14 CLINICAL STUDIES
14.1 Active, Mild to Moderate Ulcerative Colitis
Two similarly designed, randomized, double blind, placebo-controlled trials were conducted in 517 adult patients with active, mild to moderate ulcerative colitis. The study population was primarily Caucasian (80%), had a mean age of 42 years (6% age 65 years or older), and was approximately 50% male. Both studies used LIALDA doses of 2.4 g/day and 4.8 g/day administered once daily for 8 weeks except for the 2.4 g/day group in Study 1, which was given in two divided doses (1.2 g twice daily). The primary efficacy end-point in both trials was to compare the percentage of patients in remission after 8 weeks of treatment for the LIALDA treatment groups versus placebo. Remission was defined as an Ulcerative Colitis Disease Activity Index (UC-DAI) of ≤ 1, with scores of zero for rectal bleeding and for stool frequency, and a sigmoidoscopy score reduction of 1 point or more from baseline.
In both studies, the LIALDA doses of 2.4 g/day and 4.8 g/day demonstrated superiority over placebo in the primary efficacy endpoint (Table 5). Both LIALDA doses also provided consistent benefit in secondary efficacy parameters, including clinical improvement, treatment failure, clinical remission, and sigmoidoscopic improvement. LIALDA 2.4 g/day and 4.8 g/day had similar efficacy profiles.
Table 5: Patients in Remission at Week 8
| Dose |
Study 1 (n=262) n/N (%) |
Study 2 (n=255) n/N (%) |
| LIALDA 2.4 g/day | 30/88 (34.1) | 34/84 (40.5) |
| LIALDA 4.8 g/day | 26/89 (29.2) | 35/85 (41.2) |
| Placebo | 11/85 (12.9) | 19/86 (22.1) |
14.2 Maintenance of Remission in Patients with Ulcerative Colitis
A multicenter, randomized, double-blind, active comparator study was conducted in a total of 826 adult patients in remission from ulcerative colitis. The study population had a mean age of 45 years (8% age 65 years or older), were 52% male, and were primarily Caucasian (64%).
Maintenance of remission was assessed using a modified Ulcerative Colitis Disease Activity Index (UC-DAI). For this trial, maintenance of remission was based on maintaining endoscopic remission defined as a modified UC-DAI endoscopy subscore of ≤1. An endoscopy subscore of 0 represented normal mucosal appearance with intact vascular pattern and no friability or granulation. For this trial the endoscopy score definition of 1 (mild disease) was modified such that it could include erythema, decreased vascular pattern, and minimal granularity; however, it could not include friability.
Subjects were randomized in a 1:1 ratio to receive either LIALDA 2.4 g/day administered once daily or mesalamine delayed release 1.6 g/day administered as 0.8 g twice daily. The proportion of patients who maintained remission at Month 6 in this study using LIALDA 2.4 g once daily (83.7%) was similar to that seen using the comparator (mesalamine delayed release) 1.6 g/day (81.5%).
16 HOW SUPPLIED/STORAGE AND HANDLING
LIALDA is available as red-brown ellipsoidal film coated delayed-release tablets containing 1.2 g mesalamine, and debossed on one side imprinted with S476.
NDC 54092-476-12 HDPE Bottle with a child-resistant closure of 120 delayed-release tablets.
Store at room temperature 15°C to 25°C (59°F to 77°F); excursions permitted to 30°C (86°F).
See USP Controlled Room Temperature.
17 PATIENT COUNSELING INFORMATION
- Instruct patients not to take LIALDA if they have hypersensitivity to salicylates (e.g., aspirin) or other mesalamines.
- Inform patients to let their physicians know all medications they are taking and if they:
- are allergic to sulfasalazine, salicylates or mesalamine;
- are taking non-steroidal anti-inflammatory drugs (NSAIDs) or other nephrotoxic agents;
- are taking azathioprine, or 6-mercaptopurine;
- experience cramping, abdominal pain, bloody diarrhea, fever, headache or rash;
- have a history of myocarditis or pericarditis;
- have kidney or liver disease;
- have a history of stomach blockage;
- are pregnant, intend to become pregnant or are breast-feeding.
- Patients should be instructed to swallow LIALDA delayed-release tablets whole, taking care not to break the outer coating.
Manufactured for Shire US Inc., 725 Chesterbrook Blvd., Wayne, PA 19087, USA by Cosmo S.p.A., Milan, Italy. By license of Nogra Pharma Limited, Dublin, Ireland.
U.S. Patent No. 6,773,720.
© 2014 Shire US Inc.
NDC 54092-476-12 - 1.2g 120 Count Bottle
ONCE-DAILY
NDC 54092-476-12
Lialda
®
(mesalamine)
delayed release
tablets
1.2 g per tablet
Rx Only
120 Tablets
Shire

NDC 54092-476-08 Display Carton and Sample Carton with Blister Pack
ONCE-DAILY
Lialda
®
(mesalamine)
delayed release
tablets
1.2 g per tablet
Rx only
Patient Sample–Not for Sale
3 Cartons of 8 Tablets Each
Shire
NDC 54092-476-08

NDC 54092-476-01 - 1.2g 120 Count Patient Sample Bottle
ONCE-DAILY
NDC 54092-476-01
Lialda
®
(mesalamine)
delayed release
tablets
1.2 g per tablet
Rx Only
120 Tablets
Patient Sample–Not for Sale
Shire

NDC 54092-476-02 - 1.2g Carton of 12 bottles containing 120 tablets each
ONCE-DAILY
NDC 54092-476-02
Lialda
®
(mesalamine)
delayed release tablets
1.2 g per tablet
Rx only
1 carton of 12 bottles containing 120 tablets each
Shire

Lialdamesalamine TABLET, DELAYED RELEASE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||