Lisinopril
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- LISINOPRIL DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- LISINOPRIL CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- LISINOPRIL ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
WARNING: FETAL TOXICITYSee full prescribing information for complete boxed warning.
When pregnancy is detected, discontinue lisinopril as soon as possible.
Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. See Warnings: Fetal Toxicity
LISINOPRIL DESCRIPTION
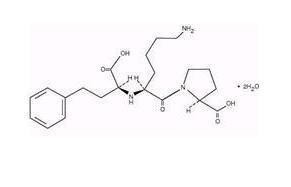
CLINICAL PHARMACOLOGY
Mechanism of Action:PRECAUTIONS). Removal of angiotensin II negative feedback on renin secretion leads to increased plasma renin activity.Pharmacokinetics and Metabolism:
DOSAGE AND ADMINISTRATION). Lisinopril can be removed by hemodialysis.
Pharmacodynamics and Clinical Effects
Hypertension:
WARNINGS). When given together with thiazide-type diuretics, the blood pressure lowering effects of the two drugs are approximately additive.
In most patients studied, onset of antihypertensive activity was seen at one hour oral administration of an individual dose of lisinopril, with peak reduction of blood pressure achieved by 6 hours. Although an antihypertensive effect was observed 24 hours after dosing with recommended single daily doses, the effect was more consistent an the mean effect was considerably larger in some studies with doses of 20 mg or more than with lower doses; however, at all doses studied, the mean antihypertensive effect was substantially smaller 24 hours after dosing than it was 6 hours after doing.
PRECAUTIONS).
DOSAGE AND ADMINISTRATION).
Heart Failure:
Acute Myocardial Infarction:
DOSAGE AND ADMINISTRATION).
ADVERSE REACTIONS - Acute Myocardial Infarction.
INDICATIONS & USAGE
Hypertension:Lisinopril tablets are indicated for the treatment of hypertension. It may be used alone as initial therapy or concomitantly with other classes of antihypertensive agents.Heart Failure:Lisinopril tablets are indicated as adjunctive therapy in the management of heart failure in patients who are not responding adequately to diuretics and digitalis.
Acute Myocardial Infarction:Lisinopril tablets are indicated for the treatment of hemodynamically stable patients within 24 hours of acute myocardial infarction, to improve survival. Patients should receive, as appropriate, the standard recommended treatments such as thrombolytics, aspirin and beta-blockers.
WARNINGS).
WARNINGS, Anaphylactoid and Possibly Related Reactions).
LISINOPRIL CONTRAINDICATIONS
WARNINGS
Anaphylactoid and Possibly Related Reactions:Head and Neck Angioedema:
Where there is involvement of the tongue, glottis or larynx, likely to cause airway obstruction, appropriate therapy, e.g., subcutaneous epinephrine solution 1:1000 (0.3 mL to 0.5 mL) and/or measures necessary to ensure a patent airway should be promptly provided (See ADVERSE REACTIONS).
Intestinal Angioedema:
Intestinal angioedema has been reported in patients treated with ACE inhibitors. These patients presented with abdominal pain (with or without nausea or vomiting); in some cases there was no prior history of facial angioedema and C-1 esterase levels were normal. The angioedema was diagnosed by procedures including abdomincal CT scan or ultrasound, or at surgery, and symptoms resolved after stopping the ACE inhibitor. Intestinal angioedema shoul dbe included in the differential diagnosis of patients on ACE inhibitors presenting with abdominal.
INDICATIONS AND USAGEandCONTRAINDICATIONS).
Anaphylactoid Reactions During Desensitization:
Anaphylactoid Reactions During Membrane Exposure:
Hypotension:
DOSAGE AND ADMINISTRATION).
PRECAUTIONS, Drug InteractionsandADVERSE REACTIONS).
Leukopenia/Neutropenia/Agranulocytosis:
Hepatic Failure:
Fetal Toxicity
Pregnancy category D:Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue lisinopril as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus.
Precautions, Pediatric Use).
PRECAUTIONS
GeneralAortic Stenosis/Hypertrophic Cardiomyopathy:
Impaired Renal Function:
Evaluation of patients with hypertension, heart failure, or myocardial infarction should always include assessment of renal function (See DOSAGE AND ADMINISTRATION).
Hyperkalemia:
In clinical trials hyperkalemia (serum potassium greater than 5.7 mEq/L) occurred in approximately 2.2% of hypertensive patients and 4.8% of patients with heart failure. In most cases these were isolated values which resolved despite continued therapy. Hyperkalemia was a cause of discontinuation oftherapy in approximately 0.1% of hypertensive patients; 0.6% of patients with heart failure and 0.1% of patients with myocardial infarction. Risk factors for the development of hyperkalemia include renal insufficiency, diabetes mellitus, and the concomitant use of potassium-sparing diuiretics, potassium supplements and/or potassium-containing salt substitutes. Hyperkalemia can cause serious, sometimes fatal, arrhythmias. Lisinopril should be used cautiously, if at all, with these agents and with frequent monitoring of serum potassium. PRECAUTIONS,Drug Interactions).
Cough:
Surgery/Anesthesia:
INFORMATION FOR PATIENTS
Angioedema:Angioedema, including laryngeal edema may occur at any time during treatment with angiotensin-converting enzyme inhibitors, including lisinopril. Patients should be so advised and told to report immediately any signs or symptoms suggesting angioedema (swelling of face, extremities, eyes, lips, tongue, difficulty in swallowing or breathing) and to take no more drug until they have consulted with the prescribing physician.Symptomatic Hypotension:Patients should be cautioned to report lightheadedness especially during the first few days of therapy. If actual syncope occurs, the patient should be told to discontinue the drug until they have consulted with the prescribing physician.
Hyperkalemia:Patients should be told not to use salt substitutes containing potassium without consulting their physician.
Hypoglycemia:Diabetic patients treated with oral antidiabetic agents or insulin starting an ACE inhibitor should be told to closely monitor for hypoglycemia, especially during the first month of combined use (SeePRECAUTIONS, Drug Interactions).
Leukopenia/Neutropenia:Patients should be told to report promptly any indication of infection (e.g., sore throat, fever) which may be a sign of leukopenia/neutropenia.
Pregnancy:Female patients of childbearing age should be told about the consequences of exposure to lisinopril during pregnancy. Discuss treatment options with women planning to become pregnant. Patients should be asked to report pregnancies to their physicians as soon as possible.
NOTE:As with many other drugs, certain advice to patients being treated with lisinopril is warranted. This information is intended to aid in the safe and effective use of this medication. It is not a disclosure of all possible adverse or intended effects.
DRUG INTERACTIONS
Hypotension - Patients on Diuretic Therapy:Patients on diuretics and especially those in whom diuretic therapy was recently instituted, may occasionally experience an excessive reduction of blood pressure after initiation of therapy with lisinopril. The possibility of hypotensive effects with lisinopril can be minimized by either discontinuing the diuretic or increasing the salt intake prior to initiation of treatment with lisinopril. If it is necessary to continue the diuretic, initiate therapy with lisinopril at a dose of 5 mg daily, and provide close medical supervision after the initial dose until blood pressure has stabilized (SeeWARNINGSandDOSAGE AND ADMINISTRATION). When a diuretic is added to the therapy of a patient receiving lisinopril, an additional antihypertensive effect is usually observed. Studies with ACE inhibitors in combination with diuretics indicate that the dose of the ACE inhibitor can be reduced when it is given with a diuretic (SeeDOSAGE AND ADMINISTRATION).Antidiabetics:Epidemiological studies have suggested that concomitant administration of ACE inhibitors and antidiabetic medicines (insulins, oral hypoglycemic agents) may cause an increased blood-glucose-lowering effect with risk of hypoglycemia. This phenomenon appeared to be more likely to occur during the first weeks of combined treatment and in patients with renal impairment. In diabetic patients treated with oral antidiabetic agents or insulin, glycemic control should be closely monitored for hypoglycemia, especially during the first month of treatment with an ACE inhibitor.
Non-Steroidal Anti-Inflammatory Agents including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors):In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, coadministration of NSAIDs, including selective COX-2 inhibitors, with ACE inhibitors, including lisinopril, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving lisinopril and NSAID therapy.
Other Agents:Lisinopril has been used concomitantly with nitrates and/or digoxin without evidence of clinically significant adverse interactions. This included post myocardial infarction patients who were receiving intravenous or transdermal nitroglycerin. No clinically important pharmacokinetic interactions occurred when lisinopril was used concomitantly with propranolol or hydrochlorothiazide. The presence of food in the stomach does not alter the bioavailability of lisinopril.
Agents Increasing Serum Potassium:Lisinopril attenuates potassium loss caused by thiazide-type diuretics. Use of lisinopril with potassium-sparing diuretics (e.g., spironolactone, eplerenone, triamterene or amiloride), potassium supplements, or potassium-containing salt substitutes may lead to significant increases in serum potassium. Therefore, if concomitant use of these agents is indicated because of demonstrated hypokalemia, they should be used with caution and with frequent monitoring of serum potassium. Potassium-sparing agents should generally not be used in patients with heart failure who are receiving lisinopril.
Lithium:Lithium toxicity has been reported in patients receiving lithium concomitantly with drugs which cause elimination of sodium, including ACE inhibitors. Lithium toxicity was usually reversible upon discontinuation of lithium and the ACE inhibitor. It is recommended that serum lithium levels be monitored frequently if lisinopril is administered concomitantly with lithium.
Gold:Nitritoid reactions (symptoms include facial flushing, nausea, vomiting and hypotension) have been reported rarely in patients on therapy with injectable gold (sodium aurothiomalate) and concomitant ACE inhibitor therapy including lisinopril.
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
NURSING MOTHERS
PEDIATRIC USE
CLINICAL PHARMACOLOGY, Pharmacokinetics and MetabolismandPharmacodynamics and Clinical Effects, andDOSAGE AND ADMINISTRATION).
GERIATRIC USE
CLINICAL PHARMACOLOGY, Pharmacodynamics and Clinical Effects,Heart FailureandCLINICAL PHARMACOLOGY, Pharmacodynamics and Clinical Effects,Acute Myocardial Infarction).
CLINICAL PHARMACOLOGY, Pharmacokinetics and Metabolism).
DOSAGE AND ADMINISTRATION).
LISINOPRIL ADVERSE REACTIONS
Hypertension
PERCENT OF PATIENTS IN CONTROLLED STUDIES
Heart Failure
Acute Myocardial Infarction
Body as a Whole:Anaphylactoid reactions (SeeWARNINGS, Anaphylactoid and Possibly Related Reactions), syncope, orthostatic effects, chest discomfort, pain, pelvic pain, flank pain, edema, facial edema, virus infection, fever, chills, malaise.
Cardiovascular:WARNINGS, Hypotension
Digestive:Pancreatitis, hepatitis (hepatocellular or cholestatic jaundice) (SeeWARNINGS, Hepatic Failure), vomiting, gastritis, dyspepsia, heartburn, gastrointestinal cramps, constipation, flatulence, dry mouth.
Hematologic:Rare cases of bone marrow depression, hemolytic anemia, leukopenia/neutropenia and thrombocytopenia.
Endocrine:Diabetes mellitus, inappropriate antidiuretic hormone secretion.
Metabolic:Weight loss, dehydration, fluid overload, gout, weight gain.
PRECAUTIONS, Drug Interactions).
Musculoskeletal:Arthritis, arthralgia, neck pain, hip pain, low back pain, joint pain, leg pain, knee pain, shoulder pain, arm pain, lumbago.
Nervous System/Psychiatric:Stroke, ataxia, memory impairment, tremor, peripheral neuropathy (e.g., dysesthesia), spasm, paresthesia, confusion, insomnia, somnolence, hypersomnia, irritability, nervousness and mood alterations (including depressive symptoms).
Respiratory System:Malignant lung neoplasms, hemoptysis, pulmonary infiltrates, bronchospasm, asthma, pleural effusion, pneumonia, eosinophilic pneumonitis, bronchitis, wheezing, orthopnea, painful respiration, epistaxis, laryngitis, sinusitis, pharyngeal pain, pharyngitis, rhinitis, rhinorrhea.
Skin:
Uritcaria, alopecia, herpes zoster, photosensitivity, skin lesions, skin infections, pemphigus, erythema, flushing, diaphoresis, cutaneous pseudolymphoma, psoriasis. Other severe skin reactions have been reported rarely, including toxic epidermal necrolysis and Stevens-Johnson syndrome; causal relationship has not been established.Special Senses:Visual loss, diplopia, blurred vision, tinnitus, photophobia, taste disturbances, olfactory disturbance.
Urogenital System:Acute renal failure, oliguria, anuria, uremia, progressive azotemia, renal dysfunction (SeePRECAUTIONSandDOSAGE AND ADMINISTRATION), pyelonephritis, dysuria, urinary tract infection, breast pain.
Miscellaneous:A symptom complex has been reported which may include a positive ANA, an elevated erythrocyte sedimentation rate, arthralgia/arthritis, myalgia, fever, vasculitis, eosinophilia and leukocytosis. Rash, photosensitivity or other dermatological manifestations may occur alone or in combination with these symptoms.
Angioedema:Angioedema has been reported in patients receiving lisinopril with an incidence higher in Black than in non-Black patients. Angioedema associated with laryngeal edema may be fatal. If angioedema of the face, extremities, lips, tongue, glottis and/or larynx occurs, treatment with lisinopril should be discontinued and appropriate therapy instituted immediately (SeeWARNINGS).
Hypotension:In hypertensive patients, hypotension occurred in 1.2% and syncope occurred in 0.1% of patients with an incidence higher in Black than in non-Black patients. Hypotension or syncope was a cause of discontinuation of therapy in 0.5% of hypertensive patients. In patients with heart failure, hypotension occurred in 5.3% and syncope occurred in 1.8% of patients. These adverse experiences were possibly dose-related (see above data from ATLAS Trial) and caused discontinuation of therapy in 1.8% of these patients in the symptomatic trials. In patients treated with lisinopril for six weeks after acute myocardial infarction, hypotension (systolic blood pressuremmHg) resulted in discontinuation of therapy in 9.7% of the patients (SeeWARNINGS).
Cough:SeePRECAUTIONS,Cough
Pediatric Patients:No relevant differences between the adverse experience profile for pediatric patients and that previously reported for adult patients were identified.
Clinical Laboratory Test Findings
Serum Electrolytes:Hyperkalemia (SeePRECAUTIONS), hyponatremia.
Creatinine, Blood Urea Nitrogen:Minor increases in blood urea nitrogen and serum creatinine, reversible upon discontinuation of therapy, were observed in about 2.0% of patients with essential hypertension treated with lisinopril alone. Increases were more common in patients receiving concomitant diuretics and in patients with renal artery stenosis (SeePRECAUTIONS). Reversible minor increases in blood urea nitrogen and serum creatinine were observed in approximately 11.6% of patients with heart failure on concomitant diuretic therapy. Frequently, these abnormalities resolved when the dosage of the diuretic was decreased.
Hemoglobin and Hematocrit:
Liver Function Tests:Rarely, elevations of liver enzymes and/or serum bilirubin have occurred (SeeWARNINGS,Hepatic Failure).
OVERDOSAGE
WARNINGS,Anaphylactoid Reactions During Membrane Exposure).
DOSAGE & ADMINISTRATION
HypertensionInitial Therapy:In patients with uncomplicated essential hypertension not on diuretic therapy, the recommended initial dose is 10 mg once a day. Dosage should be adjusted according to blood pressure response. The usual dosage range is 20 to 40 mg per day administered in a single daily dose. The antihypertensive effect may diminish toward the end of the dosing interval regardless of the administered dose, but most commonly with a dose of 10 mg daily. This can be evaluated by measuring blood pressure just prior to dosing to determine whether satisfactory control is being maintained for 24 hours. If it is not, an increase in dose should be considered. Doses up to 80 mg have been used but do not appear to give greater effect. If blood pressure is not controlled with lisinopril alone, a low dose of a diuretic may be added. Hydrochlorothiazide, 12.5 mg has been shown to provide an additive effect. After the addition of a diuretic, it may be possible to reduce the dose of lisinopril.
Diuretic Treated Patients:In hypertensive patients who are currently being treated with a diuretic, symptomatic hypotension may occur occasionally following the initial dose of lisinopril. The diuretic should be discontinued, if possible, for two to three days before beginning therapy with lisinopril tablet to reduce the likelihood of hypotension (SeeWARNINGS). The dosage of lisinopril should be adjusted according to blood pressure response. If the patient's blood pressure is not controlled with lisinopril alone, diuretic therapy may be resumed as described above.
WARNINGSandPRECAUTIONS,Drug Interactions).
PRECAUTIONS).
Dosage Adjustment in Renal Impairment:The usual dose of lisinopril (10 mg) is recommended for patients with creatinine clearance > 30 mL/min (serum creatinine of up to approximately 3 mg/dL). For patients with creatinine clearance10 mL/min30 mL/min (serum creatinine3 mg/dL), the first dose is 5 mg once daily. For patients with creatinine clearance < 10 mL/min (usually on hemodialysis) the recommended initial dose is 2.5 mg. The dosage may be titrated upward until blood pressure is controlled or to a maximum of 40 mg daily.
WARNINGS, Anaphylactoid Reactions During Membrane Exposure.
Heart Failure
WARNINGSandPRECAUTIONS, Drug Interactions). The appearance of hypotension after the initial dose of lisinopril does not preclude subsequent careful dose titration with the drug, following effective management of the hypotension.
Dosage Adjustment in Patients with Heart Failure and Renal Impairment or Hyponatremia:In patients with heart failure who have hyponatremia (serum sodium < 130 mEq/L) or moderate to severe renal impairment (creatinine clearance30 mL/min or serum creatinine > 3 mg/dL), therapy with lisinopril should be initiated at a dose of 2.5 mg once a day under close medical supervision (SeeWARNINGSandPRECAUTIONS, Drug Interactions).
Acute Myocardial Infarction
WARNINGS). If hypotension occurs (systolic blood pressure100 mmHg) a daily maintenance dose of 5 mg may be given with temporary reductions to 2.5 mg if needed. If prolonged hypotension occurs (systolic blood pressure < 90 mmHg for more than 1 hour) lisinopril tablet should be withdrawn. For patients who develop symptoms of heart failure, seeDOSAGE AND ADMINISTRATION, Heart Failure.
Dosage Adjustment in Patients With Myocardial Infarction with Renal Impairment:In acute myocardial infarction, treatment with lisinopril tablets should be initiated with caution in patients with evidence of renal dysfunction, defined as serum creatinine concentration exceeding 2 mg/dL. No evaluation of dosing adjustments in myocardial infarction patients with severe renal impairment has been performed.
Use in Elderly
Pediatric Hypertensive Patients6 years of age
CLINICAL PHARMACOLOGY, Pharmacokinetics and MetabolismandPharmacodynamicsandClinical Effects).
CLINICAL PHARMACOLOGY, Pharmacokinetics and MetabolismandPharmacodynamics and Clinical EffectsandPRECAUTIONS).
HOW SUPPLIED
WATSONon one side and405on the other side, in bottles of 30, 100, and 500.
WATon the left andSONon the right of the score and406on the other side, in bottles of 100, 500, 1000, 3000, and 5000.
WATSONand407on the periphery of one side and plain on the other side, in bottles of 30, 100, 1000, and 3000.
WATSONand408on the periphery of one side and plain on the other side, in bottles of 30, 100, 1000, and 3000.
WATSONand885on the periphery of one side and plain on the other side, in bottles of 30, 100, 1000, and 3000.
WATSONand409on the periphery of one side and plain on the other side, in bottles of 30, 100, 500, and 1000.
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION


LisinoprilLISINOPRIL TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!