Lisinopril and Hydrochlorothiazide
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- LISINOPRIL AND HYDROCHLOROTHIAZIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- LISINOPRIL AND HYDROCHLOROTHIAZIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- LISINOPRIL AND HYDROCHLOROTHIAZIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- INACTIVE INGREDIENT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
LISINOPRIL AND HYDROCHLOROTHIAZIDE DESCRIPTION
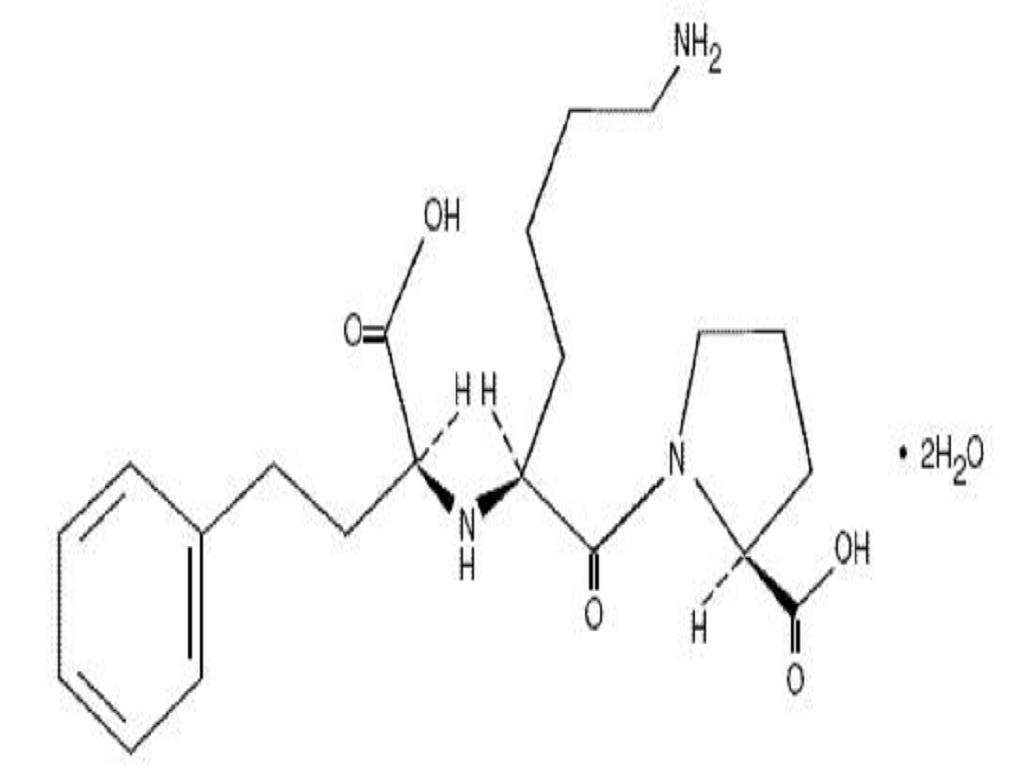
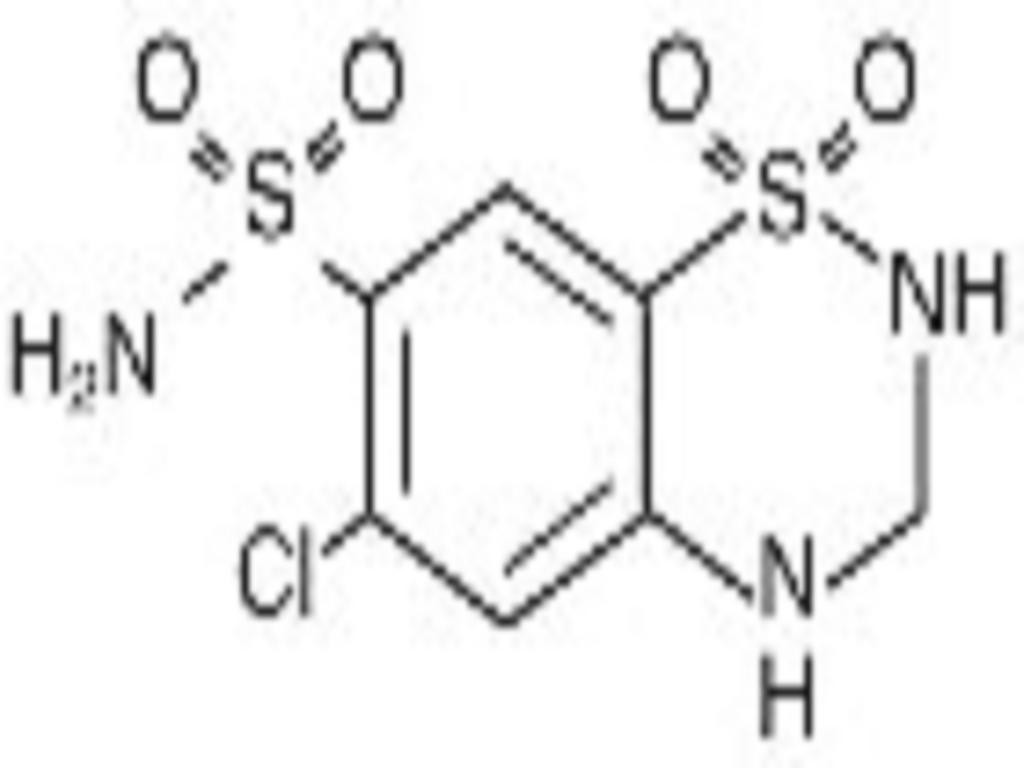
CLINICAL PHARMACOLOGY
Lisinopril-HydrochlorothiazideLisinopril
Mechanism of Action:
Pharmacokinetics and Metabolism:
Pharmacodynamics:
Hydrochlorothiazide
INDICATIONS & USAGE
LISINOPRIL AND HYDROCHLOROTHIAZIDE CONTRAINDICATIONS
WARNINGS
GeneralLisinopril
Anaphylactoid and Possibly Related Reactions:
Angioedema:
Anaphylactoid reactions during desensitization:
Anaphylactoid reactions during membrane exposure:
Hypotension and related effects
Neutropenia/Agranulocytosis:
Hepatic Failure:
Hydrochlorothiazide
Pregnancy
Lisinopril-Hydrochlorothiazide
Lisinopril
Fetal/Neonatal Morbidity and Mortality:
Hydrochlorothiazide
PRECAUTIONS
GeneralLisinopril
Aortic Stenosis/Hypertrophic Cardiomyopathy:
Impaired Renal Function:
Hyperkalemia:
Cough:
Surgery/Anesthesia:
Hydrochlorothiazide
INFORMATION FOR PATIENTS
Information for PatientsAngioedema:
Symptomatic Hypotension:
Hyperkalemia:
Neutropenia:
Pregnancy:
DRUG INTERACTIONS
LisinoprilHypotension
Patients on Diuretic Therapy:
Non-Steroidal Anti-inflammatory Agents:
Other Agents:
Agents Increasing Serum Potassium:
Lithium:
Hydrochlorothiazide
Alcohol, barbiturates, or narcotics -
Antidiabetic drugs (oral agents and insulin) -
Other antihypertensive drugs -
Cholestyramine and colestipol resins -
Corticosteroids, ACTH -
Pressor amines (e.g., norepinephrine) -
Skeletal muscle relaxants, nondepolarizing (e.g., tubocurarine)-
Lithium-
Non-Steroidal Anti-inflammatory Drugs -
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Carcinogenesis, Mutagenesis, Impairment of FertilityLisinopril and Hydrochlorothiazide
Lisinopril
Hydrochlorothiazide
PREGNANCY
NURSING MOTHERS
PEDIATRIC USE
Pediatric UseLISINOPRIL AND HYDROCHLOROTHIAZIDE ADVERSE REACTIONS
Lisinopril-HydrochlorothiazidePlacebo(n=930)(n=207)IncidenceIncidence(discontinuation)
Clinical Laboratory Test Findings
Serum Electrolytes
Serum Uric Acid, Glucose, Magnesium, Cholesterol, Triglycerides and Calcium:
Hemoglobin and Hematocrit:
Liver Function Tests:
Lisinopril -
Miscellaneous:
Fetal/Neonatal Morbidity and Mortality
Hydrochlorothiazide -
OVERDOSAGE
Lisinopril
Hydrochlorothiazide
DOSAGE & ADMINISTRATION
Dose Titration Guided by Clinical Effect
Replacement Therapy
Use in Renal Impairment
Use in Elderly
HOW SUPPLIED
INACTIVE INGREDIENT
INACTIVE INGREDIENTS:dibasic calcium phosphate
magnesium stearate
mannitol
pregelatinized starch
red iron oxide
starch(corn)
yellow iron oxide
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Lisinopril and HydrochlorothiazideLisinopril and Hydrochlorothiazide TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!