Lisinopril and Hydrochlorothiazide
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- LISINOPRIL AND HYDROCHLOROTHIAZIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- LISINOPRIL AND HYDROCHLOROTHIAZIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- LISINOPRIL AND HYDROCHLOROTHIAZIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- INACTIVE INGREDIENT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
LISINOPRIL AND HYDROCHLOROTHIAZIDE DESCRIPTION
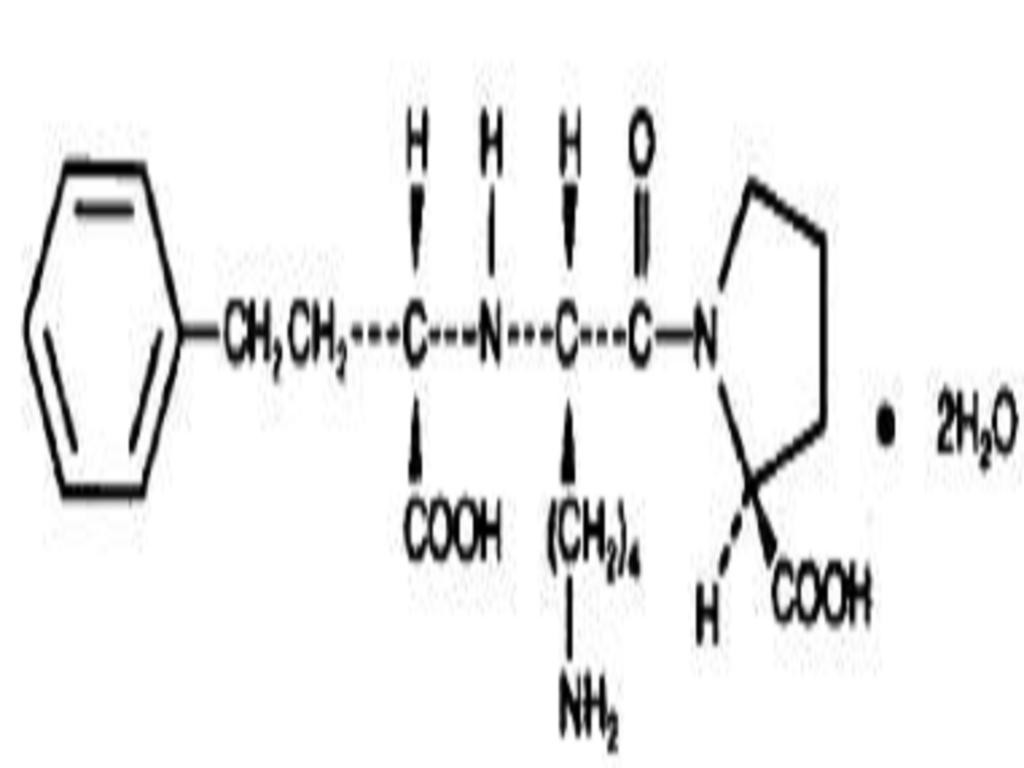
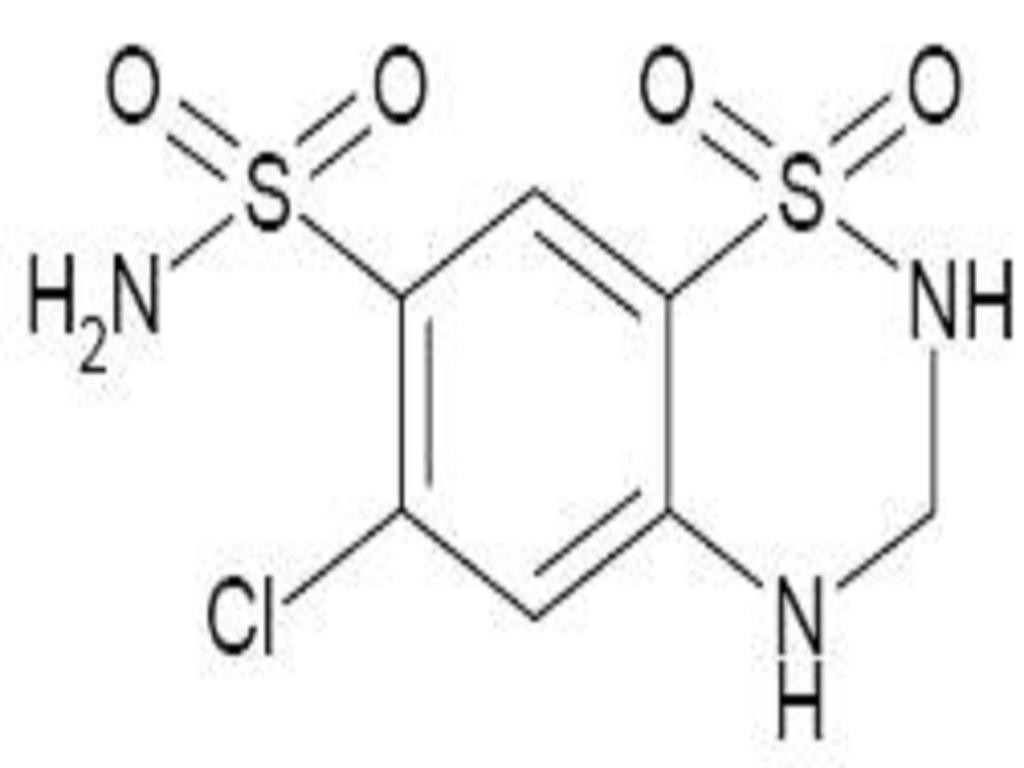
CLINICAL PHARMACOLOGY
Lisinopril and HydrochlorothiazideDOSAGE AND ADMINISTRATION
Lisinopril
Mechanism of Action
PRECAUTIONS
Pharmacokinetics and Metabolism
DOSAGE AND ADMINISTRATION
Pharmacodynamics
WARNINGS
PRECAUTIONS
Hydrochlorothiazide
INDICATIONS & USAGE
DOSAGE AND ADMINISTRATION
WARNINGS
WARNINGS, Lisinopril
LISINOPRIL AND HYDROCHLOROTHIAZIDE CONTRAINDICATIONS
WARNINGS
LisinoprilAnaphylactoid and Possibly Related Reactions
Head and Neck Angioedema
ADVERSE REACTIONS
Intestinal Angioedema
INDICATIONS AND USAGECONTRAINDICATIONS
Anaphylactoid Reactions During Desensitization
Anaphylactoid Reactions During Membrane Exposure
Hypotension and Related Effects
PRECAUTIONS, Drug InteractionsADVERSE REACTIONS
PRECAUTIONS, Drug Interactions,ADVERSE REACTIONSDOSAGE AND ADMINISTRATION
Leukopenia/Neutropenia/Agranulocytosis
Hepatic Failure
Pregnancy
Lisinopril and Hydrochlorothiazide
Lisinopril, Fetal/Neonatal Morbidity and Mortality
Lisinopril
Fetal/Neonatal Morbidity and Mortality
Hydrochlorothiazide
Teratogenic Effects
Nonteratogenic Effects
Hydrochlorothiazide
PRECAUTIONS, Drug Interactions, LisinoprilHydrochlorothiazide
PRECAUTIONS
GeneralLisinopril
Aortic Stenosis/Hypertrophic Cardiomyopathy
Impaired Renal Function
DOSAGE AND ADMINISTRATION
Hyperkalemia
PRECAUTIONS, Drug Interactions
Cough
Surgery/Anesthesia
Hydrochlorothiazide
PRECAUTIONS, Drug Interactions, Agents Increasing Serum Potassium
INFORMATION FOR PATIENTS
AngioedemaSymptomatic Hypotension
Hyperkalemia
Leukopenia/Neutropenia
Pregnancy
DRUG INTERACTIONS
LisinoprilHypotension - Patients on Diuretic Therapy
WARNINGSDOSAGE AND ADMINISTRATIONDOSAGE AND ADMINISTRATION
Non-steroidal Anti-inflammatory Agents
Other Agents
Agents Increasing Serum Potassium
Lithium
Hydrochlorothiazide
Gold
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Lisinopril and HydrochlorothiazideLisinopril
Hydrochlorothiazide
PREGNANCY
WARNINGS, Pregnancy, Lisinopril, Fetal/Neonatal Morbidity and MortalityNURSING MOTHERS
PEDIATRIC USE
Pediatric UseGERIATRIC USE
Geriatric Use
LISINOPRIL AND HYDROCHLOROTHIAZIDE ADVERSE REACTIONS
WARNINGS
Lisinopril and HydrochlorothiazidePlacebo(n=930)(n=207)IncidenceIncidence(discontinuation)
WARNINGS
WARNINGS
PRECAUTIONS - Cough
Clinical Laboratory Test Findings
PRECAUTIONS
PRECAUTIONS
PRECAUTIONS
WARNINGS, Hepatic Failure
WARNINGS, Anaphylactoid Reactions During Membrane ExposureWARNINGS, HypotensionWARNINGS, Hepatic FailurePRECAUTIONSandDOSAGE AND ADMINISTRATION), pyelonephritis, dysuria, breast pain.
Miscellaneous: A symptom complex has been reported which may include a positive ANA, an elevated erythrocyte sedimentation rate, arthralgia/arthritis, myalgia, fever, vasculitis, eosinophilia and leukocytosis. Rash, photosensitivity or other dermatological manifestations may occur alone or in combination with these symptoms.
Fetal/Neonatal Morbidity and Mortality
SeeWARNINGS - Pregnancy, Lisinopril, Fetal/Neonatal Morbidity and Mortality.
WARNINGS, Hepatic FailureWARNINGS
OVERDOSAGE
OVERDOSAGELisinopril
WARNINGS, Anaphylactoid Reactions During Membrane Exposure
Hydrochlorothiazide
DOSAGE & ADMINISTRATION
WARNINGS
To minimize dose-dependent side effects, it is usually appropriate to begin combination therapy only after a patient has failed to achieve the desired effect with monotherapy.
Dose Titration Guided by Clinical Effect
A patient whose blood pressure is not adequately controlled with either lisinopril or hydrochlorothiazide monotherapy may be switched to lisinopril and hydrochlorothiazide tablets 10 mg/12.5 mg or lisinopril and hydrochlorothiazide tablets 20 mg/12.5 mg, depending on current monotherapy dose. Further increases of either or both components should depend on clinical response with blood pressure measured at the interdosing interval to ensure that there is an adequate antihypertensive effect at that time. The hydrochlorothiazide dose should generally not be increased until 2 to 3 weeks have elapsed. After addition of the diuretic it may be possible to reduce the dose of lisinopril. Patients whose blood pressures are adequately controlled with 25 mg of daily hydrochlorothiazide, but who experience signipotassium loss with this regimen may achieve similar or greater blood-pressure control without electrolyte disturbance if they are switched to lisinopril and hydrochlorothiazide tablets 10 mg/12.5 mg.
In patients who are currently being treated with a diuretic, symptomatic hypotension occasionally may occur following the initial dose of lisinopril. The diuretic should, if possible, be discontinued for two to three days before beginning therapy with lisinopril to reduce the likelihood of hypotension. (SeeWARNINGS.) If the patient's blood pressure is not controlled with lisinopril alone, diuretic therapy may be resumed.
If the diuretic cannot be discontinued, an initial dose of 5 mg of lisinopril should be used under medical supervision for at least two hours and until blood pressure has stabilized for at least an additional hour (seeWARNINGSandPRECAUTIONS, Drug Interactions).
Concomitant administration of lisinopril and hydrochlorothiazide tablets with potassium supplements, potassium salt substitutes or potassium-sparing diuretics may lead to increases of serum potassium (seePRECAUTIONS).
Replacement Therapy
The combination may be substituted for the titrated individual components.
Use in Renal Impairment
Regimens of therapy with lisinopril and hydrochlorothiazide tablets need not take account of renal function as long as the patient's creatinine clearance is >30 mL/min/1.7 m2 (serum creatinine roughlymg/dL or 265In patients with more severe renal impairment, loop diuretics are preferred to thiazides, so lisinopril and hydrochlorothiazide tablets are not recommended (seeWARNINGS, Anaphylactoid Reactions During Membrane Exposure).
HOW SUPPLIED
INACTIVE INGREDIENT
INACTIVE INGREDIENTS:ANHYDROUS DIBASIC CALCIUM PHOSPHATE
MAGNESIUM STEARATE
MANNITOL
STARCH, CORN
STARCH, PREGELATINIZED CORN
FERRIC OXIDE RED
FERRIC OXIDE YELLOW
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Lisinopril and HydrochlorothiazideLisinopril and Hydrochlorothiazide TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!