Lisinopril and Hydrochlorothiazide
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- LISINOPRIL AND HYDROCHLOROTHIAZIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- LISINOPRIL AND HYDROCHLOROTHIAZIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- LISINOPRIL AND HYDROCHLOROTHIAZIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
USE IN PREGNANCYWhen used in pregnancy during the second and third trimesters, ACE inhibitors can cause injury and even death to the developing fetus. When pregnancy is detected, lisinopril and hydrochlorothiazide tablets should be discontinued as soon as possible. SeeWARNINGS, Pregnancy, Lisinopril, Fetal/Neonatal Morbidity and Mortality.
LISINOPRIL AND HYDROCHLOROTHIAZIDE DESCRIPTION
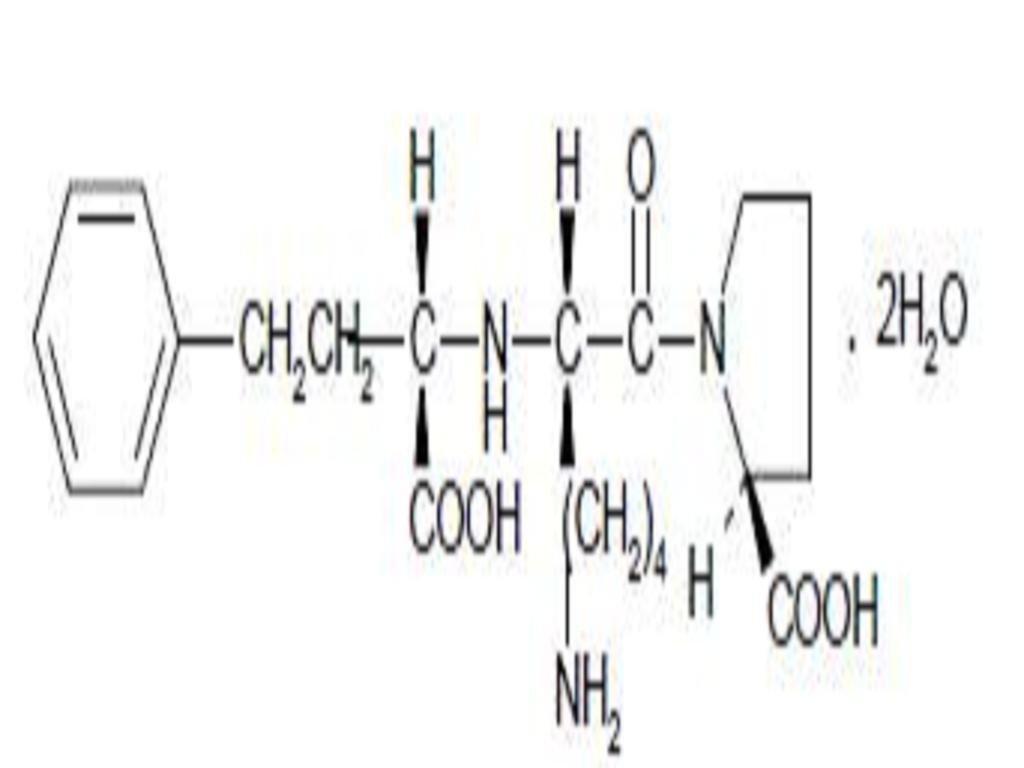
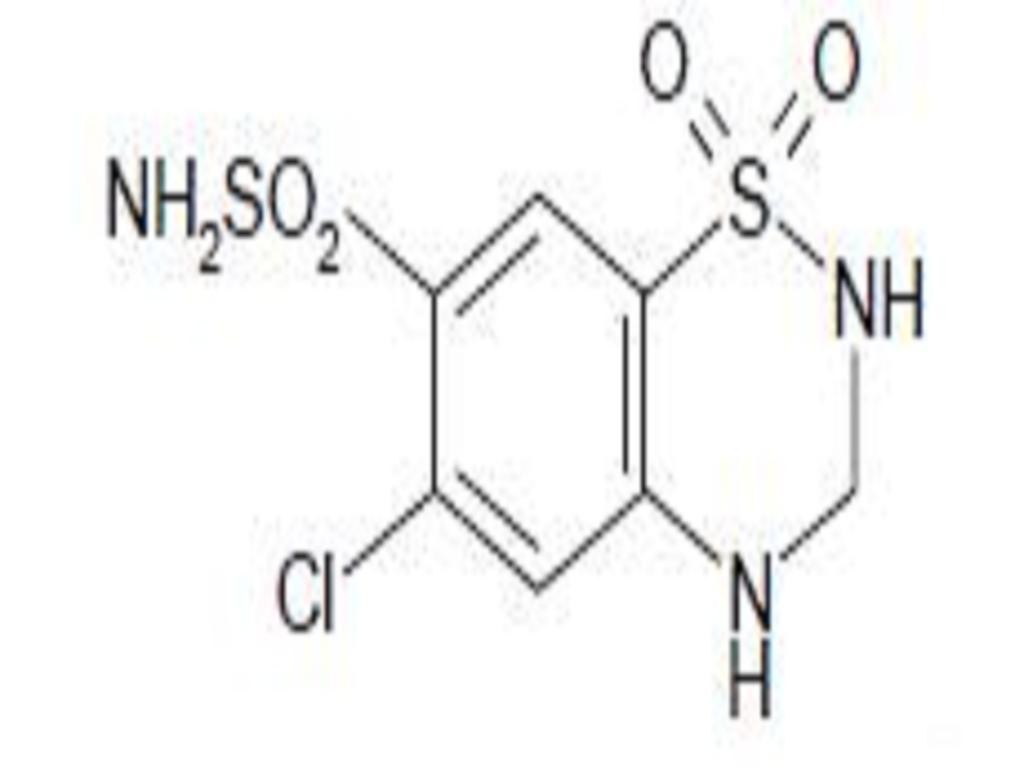
CLINICAL PHARMACOLOGY
Lisinopril HydrochlorothiazideDOSAGE AND ADMINISTRATION
Lisinopril
Mechanism of Action
PRECAUTIONS
Pharmacokinetics and Metabolism
DOSAGE AND ADMINISTRATION
Phrmacodynamics
WARNINGS
PRECAUTIONS
Hydrochlorothiazide
INDICATIONS & USAGE
DOSAGE AND ADMINISTRATION
WARNINGS
WARNINGS, Head and Neck Angioedema
LISINOPRIL AND HYDROCHLOROTHIAZIDE CONTRAINDICATIONS
WARNINGS
General
Lisinopril
Anaphylactoid and Possibly Related Reactions:
Head and Neck Angioedema:
ADVERSE REACTIONS
INDICATIONS AND USAGECONTRAINDICATIONS
Intestinal Angioedema:
Anaphylactoid reactions during desensitization:
Anaphylactoid reactions during membrane exposure:
Hypotension and Related Effects:
PRECAUTIONS , Drug InteractionsADVERSE REACTIONS
PRECAUTIONS, Drug InteractionsADVERSE REACTIONSDOSAGE AND ADMINISTRATION
Neutropenia/Agranulocytosis:
Hepatic Failure:
Hydrochlorothiazide
PRECAUTIONS, Drug Interactions, LisinoprilHydrochlorothiazide
Pregnancy
Lisinopril-Hydrochlorothiazide
Lisinopril
Hydrochlorothiazide
PRECAUTIONS
General
Lisinopril
Aortic Stenosis/Hypertrophic Cardiomyopathy:
Impaired Renal Function:
Evaluation of the hypertensive patient should always include assessment of renal functionDOSAGE AND ADMINISTRATION
Hyperkalemia:Drug Interactions
Cough:
Surgery/Anesthesia:
Hydrochlorothiazide
Drug Interactions, Agents Increasing Serum Potassium
INFORMATION FOR PATIENTS
Angioedema:Symptomatic Hypotension:
Hyperkalemia:
Neutropenia:
Pregnancy:
DRUG INTERACTIONS
Lisinopril
HypotensionPatients on Diuretic Therapy:WARNINGSDOSAGE AND ADMINISTRATIONDOSAGE AND ADMINISTRATION
Non-steroidal Anti-inflammatory Agents Including Selective Cyclooxygenase-2 (COX-2) Inhibitors:
Other Agents:
Agents Increasing Serum Potassium:
Lithium:
Gold:
Hydrochlorothiazide
Alcohol, barbiturates, or narcotics
Antidiabetic drugs (oral agents and insulin)
Other antihypertensive drugs
Cholestyramine and colestipol resins
Corticosteroids, ACTH
Pressor amines (e.g., norepinephrine)
Skeletal muscle relaxants, nondepolarizing (e.g., tubocuraine)-
Lithium -
Non-steroidal Anti-inflammatory Drugs
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Lisinopril-HydrochlorothiazideLisinopril
Hydrochlorothiazide
PREGNANCY
Teratogenic EffectWARNINGS, Pregnancy, Lisinopril, Fetal/Neonatal Morbidity and Mortality
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
DOSAGE AND ADMINISTRATION
LISINOPRIL AND HYDROCHLOROTHIAZIDE ADVERSE REACTIONS
WARNINGS
Body as a Whole:Cardiovascular:Digestive:Musculoskeletal:Nervous/Psychiatric:Respiratory:Skin:Special Senses:Urogenital:
Angioedema:WARNINGS
Hypotension:WARNINGS
Cough:PRECAUTIONS
Clinical Laboratory Test Findings
Serum Electrolytes:PRECAUTIONS
Creatinine, Blood Urea Nitrogen:PRECAUTIONS
Serum Uric Acid, Glucose, Magnesium, Cholesterol, Triglycerides and Calcium:PRECAUTIONS
Liver Function Tests:
WARNINGS, Hepatic Failure
LisinoprilBody as a WholeWARNINGS, Anaphylactoid and Possibly Related ReactionsCardiovascular:Cardiac arrest, myocardial infarction or cerebrovascular accident, possibly secondary to excessive hypotension in high risk patients (seeWARNINGS, HypotensionDigestive:Pancreatitis, hepatitis (hepatocellular or cholestatic jaundice) (seeWARNINGS, Hepatic FailureEndocrine:Diabetes mellitus, syndrome of inappropriate antidiuretic hormone secretion (SIADH)Hematologic: Metabolic: Musculoskeletal: Nervous System/Psychiatric: Respiratory: Skin: Special Senses: Urogenital:Acute renal failure, oliguria, anuria, uremia, progressive azotemia, renal dysfunction (seePRECAUTIONSandDOSAGE AND ADMINISTRATION), pyelonephritis, dysuria, breast pain.
Miscellaneous:A symptom complex has been reported which may include a positive ANA, an elevated erythrocyte sedimentation rate, arthralgia/arthritis, myalgia, fever, vasculitis, leukocytosis, eosinophilia, photosensitivity, rash, and other dermatological manifestations.
Fetal/Neonatal Morbidity and Mortality:
SeeWARNINGS, Pregnancy, Lisinopril, Fetal/Neonatal Morbidity and Mortality.
Hydrochlorothiazide
Body as a Whole: Digestive: Hematologic: Musculoskeletal: Nervous System/Psychiatric: Renal:Renal failure, renal dysfunction, interstitial nephritis (seeWARNINGSSkin: Special Senses: Hypersensitivity:Purpura, photosensitivity, urticaria, necrotizing angiitis (vasculitis and cutaneous vasculitis), respiratory distress including pneumonitis and pulmonary edema, anaphylactic reactions.
OVERDOSAGE
Lisinopril
WARNINGS, Anaphylactoid reactions during membrane exposure
Hydrochlorothiazide
DOSAGE & ADMINISTRATION
WARNINGS
To minimize dose-independent side effects, it is usually appropriate to begin combination therapy only after a patient has failed to achieve the desired effect with monotherapy.
Dose Titration Guided by Clinical Effect
A patient whose blood pressure is not adequately controlled with either lisinopril or hydrochlorothiazide monotherapy may be switched to lisinopril and hydrochlorothiazide tablets 10 mg/12.5 mg or lisinopril and hydrochlorothiazide tablets 20 mg/12.5 mg. Further increases of either or both components could depend on clinical response. The hydrochlorothiazide dose should generally not be increased until 2-3 weeks have elapsed. Patients whose blood pressures are adequately controlled with 25 mg of daily hydrochlorothiazide, but who experience significant potassium loss with this regimen, may achieve similar or greater blood pressure control with less potassium loss if they are switched to lisinopril and hydrochlorothiazide tablets 10 mg/12.5 mg. Dosage higher than lisinopril 80 mg and hydrochlorothiazide 50 mg should not be used.
Replacement Therapy
The combination may be substituted for the titrated individual components.
Use in Renal Impairment
The usual regimens of therapy with lisinopril and hydrochlorothiazide tablets need not be adjusted as long as the patient's creatinine clearance is >30 mL/min/1.73 m2 (serum creatinine approximately (3 mg/dL or 265In patients with more severe renal impairment, loop diuretics are preferred to thiazides, so lisinopril and hydrochlorothiazide tablets are not recommended (seeWARNINGS, Anaphylactoid reactions during membrane exposure).
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Lisinopril and HydrochlorothiazideLisinopril and Hydrochlorothiazide TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!