Locoid
Onset Dermatologics LLC
Precision Dermatology, Inc.
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Locoid® Lotion safely and effectively. See full prescribing information for Locoid® Lotion. Locoid® (hydrocortisone butyrate) Lotion For topical use only Initial U.S. Approval: 1982INDICATIONS AND USAGELocoid® Lotion is a corticosteroid indicated for the topical treatment of mild to moderate atopic dermatitis in patients 3 months of age and older. (1)DOSAGE AND ADMINISTRATION Locoid® Lotion is not for oral, ophthalmic, or intravaginal use. (2) Apply a thin layer to the affected skin two times daily. (2) Rub in gently. (2) Discontinue Locoid® Lotion when control is achieved. (2) Reassess diagnosis if no improvement is seen within 2 weeks. (2) Safety of Locoid® Lotion has not been established beyond 4 weeks of use. (2) DOSAGE FORMS AND STRENGTHSLotion: 0.1% (1 mg/g), supplied in bottles of 2 fl. oz. and 4 fl. oz. CONTRAINDICATIONSNone. (4)WARNINGS AND PRECAUTIONS Reversible hypothalamic-pituitary-adrenal (HPA) axis sup- pression may occur, with the potential for glucocorticosteroid insufficiency. Consider periodic evaluations for HPA axis suppression if Locoid® Lotion is applied to large surface areas or used under occlusion. If HPA axis suppression is noted, reduce the application frequency, discontinue use, or switch to a lower potency corticosteroid. (5.1, 8.4) Systemic effects of topical corticosteroids may also include manifestations of Cushing’s syndrome, hyperglycemia, and glucosuria. (5.1, 8.4) Pediatric patients may be more susceptible to systemic toxicity due to their larger skin surface-to-body-mass ratios. (5.1, 8.4) Initiate appropriate therapy if concomitant skin infections develop. (5.2) Discontinue use if irritation develops. (5.3) Side EffectsThe most common adverse reactions (greater than 1%) are HPA axis suppression and application site reactions. (6) To report SUSPECTED ADVERSE REACTIONS, contact Onset Dermatologics, LLC at 1- 800-978-5060 and/or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 LOCOID INDICATIONS AND USAGE
- 2 LOCOID DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 LOCOID CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 LOCOID ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 11 LOCOID DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Locoid® Lotion is a corticosteroid indicated for the topical treatment of mild to moderate atopic dermatitis in patients 3 months of age and older.
2 DOSAGE AND ADMINISTRATION
Locoid® Lotion is not for oral, ophthalmic, or intravaginal use. Apply a thin layer to the affected skin areas two times daily.Rub in gently.
Therapy should be discontinued when control is achieved. If no improvement is seen within 2 weeks, reassessment of the diagnosis may be necessary. The safety of Locoid® Lotion has not been established beyond 4 weeks of use.
Locoid® Lotion should not be used with occlusive dressings or applied in the diaper area unless directed by a physician.
3 DOSAGE FORMS AND STRENGTHS
Lotion: 0.1% (1 mg/g), supplied in bottles of 2 fl. oz. and 4 fl. oz.
4 CONTRAINDICATIONS
None.
5 WARNINGS AND PRECAUTIONS
5.1 Hypothalamic-pituitary-adrenal (HPA) Axis Suppression
Systemic effects of topical corticosteroids may include reversible HPA axis suppression, manifestations of Cushing’s syndrome, hyperglycemia, and glucosuria.
Studies conducted in pediatric subjects demonstrated reversible HPA axis suppression after use of Locoid® Lotion. Pediatric patients may be more susceptible than adults to systemic toxicity from equivalent doses of Locoid® Lotion due to their larger skin surface-to-body-mass ratios [see Use in Specific Populations (8.4) ].
Patients applying a topical corticosteroid to a large surface area or to areas under occlusion should be considered for periodic evaluation of the HPA axis. This may be done by using cosyntropin (ACTH1-24) stimulation testing (CST).
If HPA axis suppression is noted, the frequency of application should be reduced or the drug should be withdrawn, or a less potent corticosteroid should be substituted. Signs and symptoms of glucocorticosteroid insufficiency may occur, requiring supplemental systemic corticosteroids.
5.2 Concomitant Skin Infections
If skin infections are present or develop, an appropriate anti- fungal, antibacterial or antiviral agent should be used. If a favorable response does not occur promptly, use of Locoid® Lotion should be discontinued until the infection has been adequately controlled.
5.3 Skin Irritation
Locoid® Lotion may cause local skin adverse reactions [ see Adverse Reactions (6)].
If irritation develops, Locoid® Lotion should be discontinued and appropriate therapy instituted. Allergic contact dermatitis with corticosteroids is usually diagnosed by observing a failure to heal rather than noticing a clinical exacerbation. Such an observation should be corroborated with appropriate patch testing.
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- HPA axis suppression. This has been observed in pediatric subjects using Locoid Lotion [see Warnings and Precautions (5.1) and Use in Specific Populations (8.4)]
- Concomitant skin infections [ see Warnings and Precautions (5.2)]
- Skin irritation [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
The safety data derived from Locoid® Lotion clinical trials reflect exposure to Locoid® Lotion twice daily for up to four weeks in separate clinical trials involving pediatric subjects 3 months to 18 years of age and adult subjects 18 years and older with mild to moderate atopic dermatitis. Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed cannot be directly compared to rates in other clinical trials and may not reflect the rates observed in clinical practice.
Adverse reactions shown in the tables below include those for which there is some basis to believe there is a causal relationship to Locoid® Lotion. Although the rates of application site reactions in the vehicle group were greater than those in the Locoid® group in both studies, these rates are included in the tables (Table 1 and Table 2) because skin irritation is a known adverse reaction of topical corticosteroids.
|
|
Locoid Lotion(n=139) n(%) |
Vehicle(n=145) n(%) |
|
Application site reactions, including application site burning, pruritus, dermatitis, erythema, eczema, inflammation, or irritation |
2 (1) |
20 (14) |
|
Infantile acne |
1 (1) |
0 (0) |
|
Skin depigmentation |
1 (1) |
0 (0) |
|
|
Locoid Lotion(n=151) n (%) |
Vehicle(n=150) n (%) |
|
Application site reactions, including application site burning, dermatitis, eczema, erythema, or pruritus |
5 (3) |
7 (5) |
The following additional local adverse reactions have been reported infrequently with topical corticosteroids, and they may occur more frequently with the use of occlusive dressings and higher potency corticosteroids. These reactions included: irritation, folliculitis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, skin atrophy, striae, miliaria and telangiectasia.
7 DRUG INTERACTIONS
There are no known drug interactions with Locoid® Lotion.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C. Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Some corticosteroids have been shown to be teratogenic after dermal application in laboratory animals.
There are no adequate and well-controlled studies in pregnant women. Therefore, Locoid® Lotion should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Note: The animal multiples of human exposure calculations in this label were based on body surface area comparisons for an adult (i.e., mg/m2/day dose comparisons) assuming 100% human percutaneous absorption of a maximum topical human dose (MTHD) for hydrocortisone butyrate lotion (25 g lotion).
Systemic embryofetal development studies were conducted in rats and rabbits. Subcutaneous doses of 0.6 mg/kg/day, 1.8 mg/kg/day and 5.4 mg/kg/day hydrocortisone butyrate were administered to pregnant female rats during gestation days 6 – 17. In the presence of maternal toxicity, fetal effects noted at 5.4 mg/kg/day (2X MTHD) included an increased incidence of ossification variations and unossified sternebra. No treatment related effects on embryofetal toxicity or teratogenicity were noted at doses of 5.4 mg/kg/day and 1.8 mg/kg/day, respectively (2X MTHD and 0.7X MTHD, respectively).
Subcutaneous doses of 0.1 mg/kg/day, 0.2 mg/kg/day and 0.mg/kg/day hydrocortisone butyrate were administered to pregnant female rabbits during gestation days 7 – 20. An increased incidence of abortion was noted at 0.3 mg/kg/day (0.2X MTHD). In the absence of maternal toxicity, a dose dependent decrease in fetal body weight was noted at doses greater than or equal to 0.1 mg/kg/day (0.1X MTHD). Additional indicators of embyrofetal toxicity (reduction in litter size, decreased number of viable fetuses, increased post-implantation loss) were noted at doses greater than or equal to 0.2 mg/kg/day (0.2X MTHD). Additional fetal effects noted in this study included delayed ossification noted at doses greater than or equal to0.1 mg/kg/day and an increased incidence of fetal malformations (primarily skeletal malformations) noted at doses greater than or equal to0.2 mg/kg/day. A dose at which no treatment related effects on embryofetal toxicity or teratogenicity were observed was not established in this study.
Additional systemic embryofetal development studies were conducted in rats and mice. Subcutaneous doses of 0.1 mg/kg/day and 9 mg/kg/day hydrocortisone butyrate were administered to pregnant female rats during gestation days 9 – 15. In the presence of mater nal toxicity, an increase in fetal deaths and fetal resorptions and an increase in the number of ossifications in caudal vertebrae were noted at a dose of 9 mg/kg/day (3X MTHD). No treatment related effects on embryofetal toxicity or teratogenicity were noted at 0.1 mg/kg/day (0.1X MTHD).
Subcutaneous doses of 0.2 mg/kg/day and 1 mg/kg/day hydrocortisone bu tyrate were administered to pregnant female mice during gestation days 7 – 13. In the absence of maternal toxicity, an increased number of cervical ribs and one fetus with clubbed legs were noted at a dose of 1 mg/kg/day (0.2X MTHD). No treatment related effects on embryofetal toxicity or teratogenicity were noted at doses of 1 mg/kg/day and 0.2 mg/kg/day, respectively (0.2X MTHD and 0.1X MTHD, respectively).
No topical embryofetal development studies were conducted with hydrocortisone butyrate lotion. However, topical embryofetal development studies were conducted in rats and rabbits with a hydrocortisone butyrate ointment formulation. Topical doses of 1% and 10% hydrocortisone butyrate ointment were administered to pregnant female rats during gestation days 6 – 15 or pregnant female rabbits during gestation days 6 – 18. A dose-dependent increase in fetal resorptions was noted in rabbits (0.2 – 2X MTHD) and fetal resorptions were noted in rats at the 10% hydrocortisone butyrate ointment dose (80X MTHD). No treatment related effects on embyrofetal toxicity were noted at the 1% hydrocortisone bu- tyrate ointment dose in rats (8 MTHD). A dose at which no treatment related effects on embryofetal toxicity were observed in rabbits after topical administration of hydrocortisone butyrate ointment was not established in this study. No treatment related effects on teratogenicity were noted at a dose of 10% hydrocorti- sone butyrate ointment in rats or rabbits (80X MTHD and 2X MTHD, respectively).
A peri- and post-natal development study was
conducted in rats. Subcutaneous doses of 0.6, mg/kg/day 1.8 mg/kg/day and 5.4
mg/kg/day hydrocorti sone butyrate were administered to pregnant female rats from
gestation day 6 – lactation day 20. In the presence of maternal toxicity, a
dose dependent decrease in fetal weight was noted at doses greater than or equal
to1.8 mg/kg/day (0.7X MTHD). No treatment related effects on fetal toxicity were
noted at 0.6 mg/kg/day (0.2X MTHD). A delay in sexual maturation was
noted at 5.4 mg/kg/day (2X MTHD). No treatment related
effects on sexual maturation were noted at 1.8 mg/kg/day. No treatment related
effects on behavioral development or subsequent reproductive performance were
noted at 5.4 mg/kg/day.
8.3 Nursing Mothers
Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. Because many drugs are excreted in human milk, caution should be exercised when Locoid Lotion is administered to a nursing woman.
8.4 Pediatric Use
Safety and efficacy in pediatric patients below 3 months of age have not been established.
Because of higher skin surface-to-body-mass ratios, pediatric patients are at a greater risk than adults of HPA axis suppression when they are treated with topical corticosteroids. They are there- fore also at a greater risk of glucocorticosteroid insufficiency after withdrawal of treatment and of Cushing’s syndrome while on treatment.
Eighty-four (84) pediatric subjects (3 months to less than 18 years of age) with moderate to severe atopic dermatitis affecting at least 25% of body surface area (BSA) treated with Locoid® Lotion three times daily for up to 4 weeks were assessed for HPA axis suppression. The disease severity (moderate to severe atopic dermatitis) and the dosing regimen (three times daily) in this HPA axis study were different from the subject population (mild to moderate atopic dermatitis) and the dosing regimen (two times daily) for which Locoid® lotion is indicated. Seven of the 82 evaluable subjects (8.5%) demonstrated laboratory evidence of suppression, where the sole criterion for defining HPA axis suppression was a serum cortisol level of less than or equal to 18 micrograms per deciliter after cosyntropin stimulation. Suppressed subjects ranged in age from 1 to 12 years and, at the time of enrollment, had 35% to 90% BSA involvement. These subjects did not develop any other signs or symptoms of HPA axis suppression. At the first follow up visit, approximately one month after the conclusion of treatment, cosyntropin stimulation results of all subjects had returned to normal, with the exception of one subject. This last subject recovered adrenal function by the second post treatment visit, 55 days post-treatment.
Cushing’s syndrome, linear growth
retardation, delayed weight gain, and intracranial hypertension have also been
reported in pediatric patients receiving topical corticosteroids. Manifestations
of adrenal suppression in pediatric patients include low plasma cortisol levels to
an absence of response to ACTH stimulation. Manifestations of intracranial
hypertension include bulging fonta- nelles, headaches, and bilateral
papilledema.
8.5 Geriatric Use
Clinical studies of Locoid® Lotion did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
11 DESCRIPTION
Locoid® Lotion contains hydrocortisone butyrate, a non- fluorinated hydrocortisone ester [Pregn-4-ene-3, 20-dione, 11, 21-dihydroxy-17- [(1-oxobutyl) oxy (11β)-] for topical dermatologic use. The topical corticosteroids constitute a class of primarily synthetic steroids used as anti-inflammatory and antipruritic agents.
Chemically, hydrocortisone butyrate is C25H36O6. It has the following structural formula:
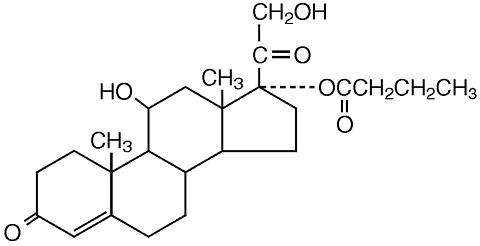
Hydrocortisone butyrate is a white to practically white powder with a molecular weight of 432.56. It is practically insoluble in water, slightly soluble in ether, soluble in methanol, in alcohol, and in acetone, and freely soluble in chloroform.
Each gram of Locoid® Lotion contains 1 mg hydrocortisone butyrate in a base consisting of purified water, light mineral oil, cetostearyl alcohol, white petrolatum, ceteth-20, citric acid, sodium citrate, safflower oil and butylated hydroxytoluene (BHT), with propylparaben and butylparaben as preservatives.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Topical corticosteroids share anti-inflammatory, antipruritic, and vasoconstrictive properties. The mechanism of the anti-inflammatory activity of the topical corticosteroids is unclear. However, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipo- cortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor, arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2.
12.3 Pharmacokinetics
No studies were conducted to determine the pharmacokinetics of Locoid® Lotion.
The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle, the integrity of the epidermal barrier, and the use of occlusive dressings.
Topical corticosteroids can be absorbed through normal in- tact skin. Inflammation and/or other disease processes in the skin, occlusive dressings, or widespread application may increase percutaneous absorption.
Once absorbed through the skin, topical corticosteroids are handled through pharmacokinetic pathways similar to systemically administered corticosteroids.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies were conducted to determine the photococarcinogenic or dermal carcinogenic potential of Locoid® Lotion.
Hydrocortisone butyrate revealed no evidence of mutagenic or clastogenic potential based on the results of two in vitro genotoxicity tests (Ames test and L5178Y/TK+ mouse lymphoma assay) and one in vivo genotoxicity test (mouse micronucleus assay).
No evidence of impairment of fertility or effect on mating performance was observed in a fertility and general reproductive performance study conducted in male and female rats at subcutaneous doses up to and including 1.8 mg/kg/day (0.7X MTHD). Mild effects on maternal animals, such as reduced food consumption and a subsequent reduction in body weight gain, were seen at doses greater than or equal to 0.6 mg/kg/day (0.2X MTHD).
14 CLINICAL STUDIES
In a multicenter, randomized, vehicle-controlled trial of 284 pediatric subjects 3 months to 18 years of age with mild to moderate atopic dermatitis, Locoid® Lotion or vehicle was applied twice daily for up to four weeks. Treatment success was assessed at day 29 (after 28 days of treatment) and was defined as the proportion of patients who achieved both “clear” or “almost clear” and at least a two grade improvement from baseline on a 5-point Physician’s Global Assessment (PGA) scale.
Study results are shown in Table 3.
|
|
Locoid Lotion (n=139) |
Vehicle (n=145) |
|
Number (%) successes |
68 (49%) |
35 (24%) |
Another multicenter, randomized, double-blind study com- pared twice daily treatment with Locoid® Lotion (n=151) to vehicle (n=150) for three or four weeks in adult subjects (ages 18 years or older), including those mild or moderate atopic dermatitis. Results favored Locoid® Lotion over vehicle.
16 HOW SUPPLIED/STORAGE AND HANDLING
Locoid® Lotion: 0.1% (1mg/g, supplied in bottles of 2 fl. oz. (NDC 16781-392-02) and 4 fl. oz. (NDC 16781-392-04)
St o rage and Handling
- Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature]
- Protect from freezing.
- Keep out of the reach of children.
- Keep bottle closed when not in use.
17 PATIENT COUNSELING INFORMATION
17.1 Instructions for Use
Patients using Locoid® Lotion should receive the following information and instructions:
- Apply a thin layer to the affected skin two times daily.
- Rub in gently.
- Discontinue Locoid® Lotion when control is achieved.
- Do not use for longer than 4 weeks.
- Avoid contact with the eyes.
- Do not bandage, otherwise cover, or wrap the affected skin area so as to be occlusive unless directed by your physician.
- Do not use Locoid® Lotion in the diaper area, as diapers or plastic pants may constitute occlusive dressings.
- Do not use Locoid® Lotion on the face, underarms, or groin areas unless directed by your physician.
- If no improvement is seen within 2 weeks, contact your physician.
- Do not use other corticosteroid-containing products while using Locoid® Lotion without first consulting your physician.

P/N 2638 Rev. 0
Locoid Lotion 2 fluid oz carton
Locoid Lotion 4 fluid oz carton
LocoidHYDROCORTISONE BUTYRATE LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||