Lorazepam
FULL PRESCRIBING INFORMATION: CONTENTS*
- LORAZEPAM DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- LORAZEPAM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- ESSENTIAL LABORATORY TESTS
- CLINICALLY SIGNIFICANT DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS
- PREGNANCY
- NURSING MOTHERS
- GERIATRIC USE
- LORAZEPAM ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
LORAZEPAM DESCRIPTION
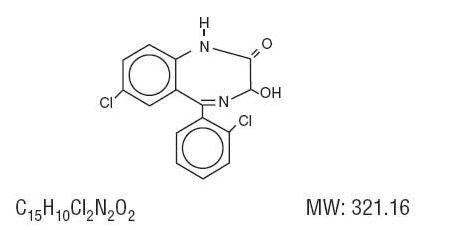
CLINICAL PHARMACOLOGY
INDICATIONS & USAGE
LORAZEPAM CONTRAINDICATIONS
WARNINGS
PRECAUTIONS, Clinically Significant Drug Interactions)
Physical And Psychological Dependence
PRECAUTIONS
INFORMATION FOR PATIENTS
ESSENTIAL LABORATORY TESTS
CLINICALLY SIGNIFICANT DRUG INTERACTIONS
CARCINOGENESIS & MUTAGENESIS
PREGNANCY
NURSING MOTHERS
GERIATRIC USE
ADVERSE REACTIONS).CLINICAL PHARMACOLOGY).
DOSAGE AND ADMINISTRATION).
LORAZEPAM ADVERSE REACTIONS
OVERDOSAGE
Symptoms
MANAGEMENT
The prescriber should be aware of a risk of seizure in association with flumazenil treatment, particularly in long-term benzodiazepine users and in cyclic antidepressant overdose.The complete flumazenil package insert includingCONTRAINDICATIONS,WARNINGS, andPRECAUTIONSshould be consulted prior to use.
DOSAGE & ADMINISTRATION
HOW SUPPLIED
240over0.5on one side andWATSONon the other side, supplied in bottles of 100, 500 and 1000.
1 mg: white, scored, round flat faced beveled edge, debossed with241over1on one side andWATSONon the other side, supplied in bottles of 100, 500 and 1000.
2 mg: white, scored, round flat faced beveled edge, debossed with242over2on one side andWATSONon the other side, supplied in bottles of 100, 500 and 1000.
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

LorazepamLORAZEPAM TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!