Losartan Potassium
FULL PRESCRIBING INFORMATION: CONTENTS*
- USE IN PREGNANCY
- LOSARTAN POTASSIUM DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- LOSARTAN POTASSIUM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- LOSARTAN POTASSIUM ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PATIENT INFORMATION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
USE IN PREGNANCY
When used in pregnancy during the second and third trimesters, drugs that act directly on the renin-angiotensin system can cause injury and even death to the developing fetus.When pregnancy is detected, losartan potassium tablets should be discontinued as soon as possible. SeeWARNINGS,Fetal/Neonatal Morbidity and Mortality.LOSARTAN POTASSIUM DESCRIPTION

CLINICAL PHARMACOLOGY
Mechanism of ActionPharmacokinetics
Special Populations
Table 1 Pharmacokinetic Parameters in Hypertensive Adults and Children Age 6 to 16 Following Multiple Dosing
DOSAGE AND ADMINISTRATION,Preparation of Suspension).
DOSAGE AND ADMINISTRATION).
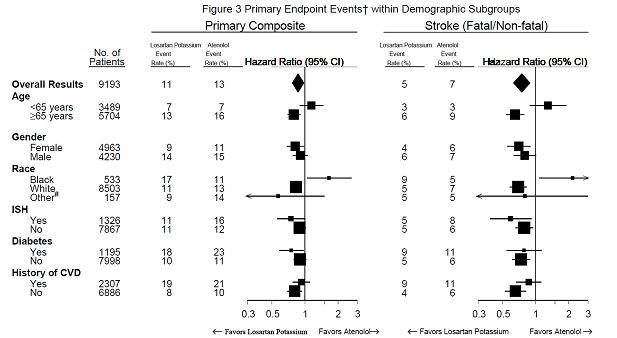
PRECAUTIONS,RaceandCLINICAL PHARMACOLOGY,Pharmacodynamics and Clinical Effects,Reduction in the Risk of Stroke,Race).
WARNINGS,HypotensionVolume-Depleted PatientsandDOSAGE AND ADMINISTRATION).
DOSAGE AND ADMINISTRATION).
Drug Interactions
Pharmacodynamics and Clinical Effects
DOSAGE AND ADMINISTRATION,Preparation of Suspension). The majority of the children had hypertension associated with renal and urogenital disease. The sitting diastolic blood pressure (SiDBP) on entry into the study was higher than the 95th percentile level for the patient's age, gender, and height. At the end of three weeks, losartan reduced systolic and diastolic blood pressure, measured at trough, in a dose-dependent manner. Overall, the two higher dosees (25 to 50 mg in patients < 50 kg; 50 to 100 mg in patients greater than or equal to 50 kg). The lowest dose, corresponding to an average daily dose of 0.07 mg/kg, did not appear to offer consistent antihypertensive efficacy. When patients were randomized to continue losartan at the two higher doses or to placebo after 3 weeks of therapy, trough diastolic blood pressure rose in patients on placebo between 5 and 7 mmHg more than patients randomized to continuing losartan. When the low dose of losartan was randomly withdrawn, the rise in trough diastolic blood pressure was the same in patients receiving placebo and in those continuing losartan, again suggesting that the lowest dose did not have significant antihypertensive efficacy. Overall, no significant differences in the overall antihypertensive effect of losartan were detected when the patients were analyzed according to age (<, greater than or equal to 12 years old) or gender. While blood pressure was reduced in all racial subgroups examined, too few non-White patients were enrolled to compare the dose-response of losartan in the non-White subgroup.

Figure 1. Kaplan-Meier estimates of the primary endpoint of time to cardiovascular death, nonfatal stroke, or nonfatal myocardial infarction in the groups treated with losartan potassium and atenolol. The Risk Reduction is adjusted for baseline Framingham risk score and level of electrocardiographic left ventricular hypertrophy.

Figure 2. Kaplan-Meier estimates of the time to fatal/nonfatal stroke in the groups treated with losartan potassium and atenolol. The Risk Reduction is adjusted for baseline Framingham risk score and level of electrocardiographic left ventricular hypertrophy.
Table 2 Incidence of Primary Endpoint Events
Nephropathy in Type 2 Diabetic Patients

Figure 4. Kaplan-Meier curve for the primary composite endpoint of doubling of serum creatinine, end stage renal disease (need for dialysis or transplantation) or death.
Table 3 Incidence of Primary Endpoint Events
Incidence Risk
Reduction 95%
CI p-Value Losartan Placebo
Table 4: Efficacy Outcomes within Demographic Subgroups
No. of Patients Losartan potassium
Event Rate
% Placebo
Event Rate
% Hazard Ratio
(95% CI) Losartan potassium Event Rate
% Placebo
Event Rate
% Hazard Ratio
(95% CI)
INDICATIONS & USAGE
HypertensionHypertensive Patients with Left Ventricular Hypertrophy
PRECAUTIONS, RaceandCLINICAL PHARMACOLOGY, Pharmacodynamics and Clinical Effects, Reduction in the Risk of Stroke, Race).
Nephropathy in Type 2 Diabetic Patients
CLINICAL PHARMACOLOGY, Pharmacodynamics and Clinical Effects).
LOSARTAN POTASSIUM CONTRAINDICATIONS
WARNINGS
Fetal/Neonatal Morbidity and MortalityHypotensionVolume-Depleted Patients
DOSAGE AND ADMINISTRATION).
PRECAUTIONS
GeneralHypersensitivity:
ADVERSE REACTIONS,Post-Marketing Experience.
Impaired Hepatic Function
DOSAGE AND ADMINISTRATIONandCLINICAL PHARMACOLOGY,Pharmacokinetics).
Impaired Renal Function
Electrolyte Imbalance
ADVERSE REACTIONS).
Information for Patients
PRECAUTIONS, Drug Interactions).
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors)
Carcinogenesis, Mutagenesis, Impairment of Fertility
Pregnancy
Nursing Mothers
Pediatric Use
Pharmacodynamics and Clinical EffectsandDOSAGE AND ADMINISTRATION).
Geriatric Use
Race
CLINICAL PHARMACOLOGY, Pharmacodynamics and Clinical Effects; Reduction in the Risk of Stroke.)
LOSARTAN POTASSIUM ADVERSE REACTIONS
HypertensionLosartan (n=1075)
Incidence
% Placebo (n=334)
Incidence
% Musculoskeletal Nervous System/Psychiatric Respiratory
Hypertensive Patients with Left Ventricular Hypertrophy
Nephropathy in Type 2 Diabetic Patients
Body as a Whole
Asthenia/Fatigue
Chest Pain
Fever
Infection
Influenza-like disease
Trauma
Cardiovascular
Hypotension
Orthostatic hypotension
Digestive
Diarrhea
Dyspepsia
Gastritis
Endocrine
Diabetic neuropathy
Diabetic vascular disease
Eyes, Ears, Nose and Throat
Cataract
Sinusitis
Hemic
Anemia
Metabolic and Nutrition
Hyperkalemia
Hypoglycemia
Weight gain
Musculoskeletal
Back pain
Leg pain
Knee pain
Muscular weakness
Nervous System
Hypesthesia
Respiratory
Bronchitis
Cough
Skin
Cellulitis
Urogenital
Urinary tract infection
Post-Marketing Experience
Laboratory Test Findings
OVERDOSAGE
DOSAGE & ADMINISTRATION
Adult Hypertensive PatientsWARNINGS,HypotensionVolume-Depleted Patients) and patients with a history of hepatic impairment (seePRECAUTIONS, General). Losartan potassium tablets, USP can be administered once or twice daily with total daily doses ranging from 25 mg to 100 mg.
CLINICAL PHARMACOLOGY,Pharmacodynamics and Clinical Effects, Hypertension).
CLINICAL PHARMACOLOGY,Pharmacodynamics and Clinical Effects, Hypertension).
Pediatric Hypertensive Patients greater than or equal to 6 years of age
CLINICAL PHARMACOLOGY,Pharmacokinetics,Special PopulationsandPharmacodynamics and Clinical EffectsandWARNINGS,HypotensionVolume-Depleted Patients.
CLINICAL PHARMACOLOGY,Pharmacokinetics,Special Populations,Pharmacodynamics and Clinical EffectsandPRECAUTIONS).
Preparation of Suspension (for 200 mL of a 2.5 mg/mL suspension)
Hypertensive Patients with Left Ventricular Hypertrophy
CLINICAL PHARMACOLOGY, Pharmacodynamics and Clinical Effects, Reduction in the Risk of Stroke).
CLINICAL PHARMACOLOGY, Pharmacodynamics and Clinical Effects, Nephropathy in Type 2 Diabetic Patients). Losartan potassium tablets, USP may be administered with insulin and other commonly used hypoglycemic agents (e.g., sulfonylureas, glitazones and glucosidase inhibitors).
HOW SUPPLIED
STORAGE AND HANDLING
PATIENT INFORMATION
Losartan Potassium Tablets, USP25 mg, 50 mg and 100 mg
Rx only
Do not take losartan potassium tablets, USP if you are pregnant or plan to become pregnant. Losartan potassium tablets, USP can harm your unborn baby causing injury and even death. Stop taking losartan potassium tablets, USP if you become pregnant and call your doctor right away.If you plan to become pregnant, talk to your doctor about other treatment options before taking losartan potassium tablets, USP .
High Blood Pressure (hypertension).Blood pressure is the force in your blood vessels when your heart beats and when your heart rests. You have high blood pressure when the force is too much. Losartan potassium tablets, USP can help your blood vessels relax so your blood pressure is lower.
Left Ventricular Hypertrophy (LVH)is an enlargement of the walls of the left chamber of the heart (the heartmain pumping chamber). LVH can happen from several things. High blood pressure is the most common cause of LVH.
Type 2 Diabetes with Nephropathy.Type 2 diabetes is a type of diabetes that happens mainly in adults. If you have diabetic nephropathy it means that your kidneys do not work properly because of damage from the diabetes.
Do not take losartan potassium tablets, USP if you are allergic to any of the ingredients of losartan potassium tablets, USP.Seethe end of this leaflet for a complete list of ingredients in losartan potassium tablets, USP.
are pregnant or planning to become pregnant.Seeis the most important information I should know about losartan potassium tablets, USP ?
Injury or death of unborn babies. Seeis the most important information I should know about losartan potassium tablets, USP?
Allergic reaction.Symptoms of an allergic reaction are swelling of the face, lips, throat or tongue. Get emergency medical help right away and stop taking losartan potassium tablets, USP.
Low blood pressure (hypotension).Low blood pressure may cause you to feel faint or dizzy. Lie down if you feel faint or dizzy. Call your doctor right away.
For people who already have kidney problems, you may see a worsening in how well your kidneys work.Call your doctor if you get swelling in your feet, ankles, or hands, or unexplained weight gain.
nota complete list of side effects. For a complete list, ask your doctor or pharmacist.
Keep losartan potassium tablets, USP and all medicines out of the reach of children.
General information about losartan potassium tablets, USP.
Active ingredients:losartan potassium, USP
Inactive ingredients:colloidal silicon dioxide, croscarmellose sodium, ferric oxide red, hydroxypropyl cellulose, magnesium stearate, silicon dioxide, silicified microcrystalline cellulose, stearic acid, talc, titanium dioxide, and triethyl citrate.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Rx only
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION


Losartan PotassiumLOSARTAN POTASSIUM TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!