Losartan Potassium
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- LOSARTAN POTASSIUM DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- LOSARTAN POTASSIUM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- LOSARTAN POTASSIUM ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- INFORMATION FOR PATIENTS
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
USE IN PREGNANCYWhen used in pregnancy during the second and third trimesters, drugs that act directly on the renin-angiotensin system can cause injury and even death to the developing fetus. WARNINGS, Fetal/Neonatal Morbidity and Mortality
LOSARTAN POTASSIUM DESCRIPTION
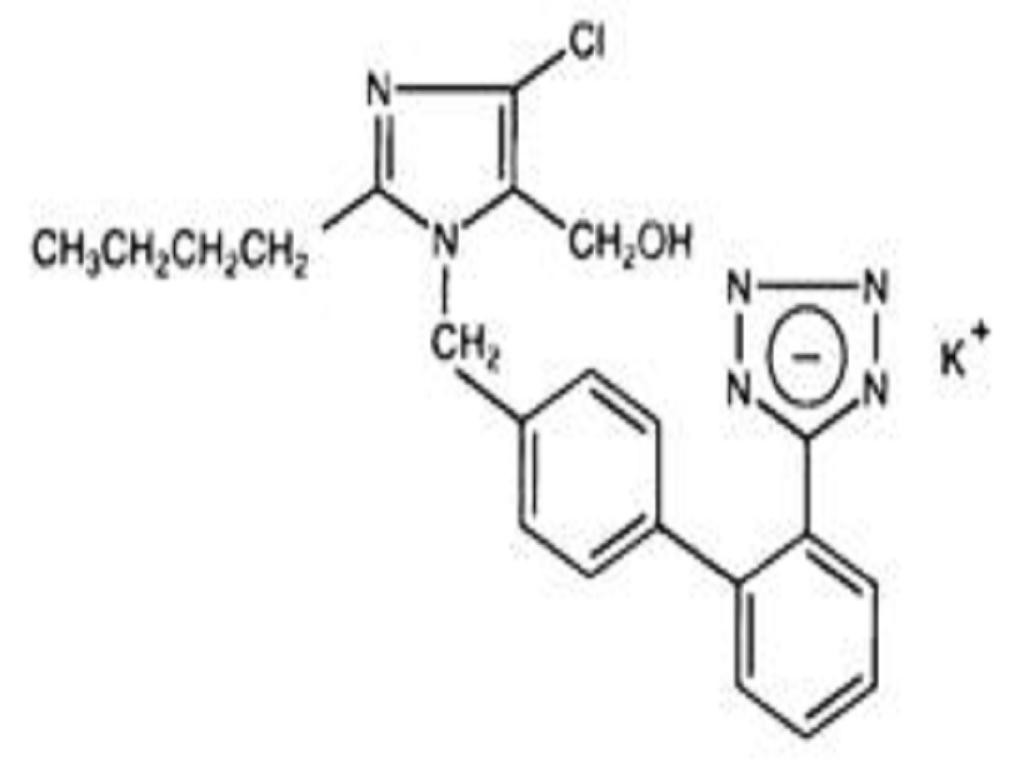
CLINICAL PHARMACOLOGY
DOSAGE AND ADMINISTRATION
DOSAGE AND ADMINISTRATION
PRECAUTIONSRaceCLINICAL PHARMACOLOGYPharmacodynamics and Clinical EffectsReduction in the Risk of StrokeRace
WARNINGSHypotensionVolume-Depleted PatientsDOSAGE AND ADMINISTRATION
DOSAGE AND ADMINISTRATION
Adult Hypertension
Pediatric Hypertension
DOSAGE AND ADMINISTRATION
Table 2Table 2).

Figure 1. Kaplan-Meier estimates of the primary endpoint of time to cardiovascular death, nonfatal stroke, or nonfatal myocardial infarction in the groups treated with losartan potassium tablets and atenolol. The Risk Reduction is adjusted for baseline Framingham risk score and level of electrocardiographic left ventricular hypertrophy.
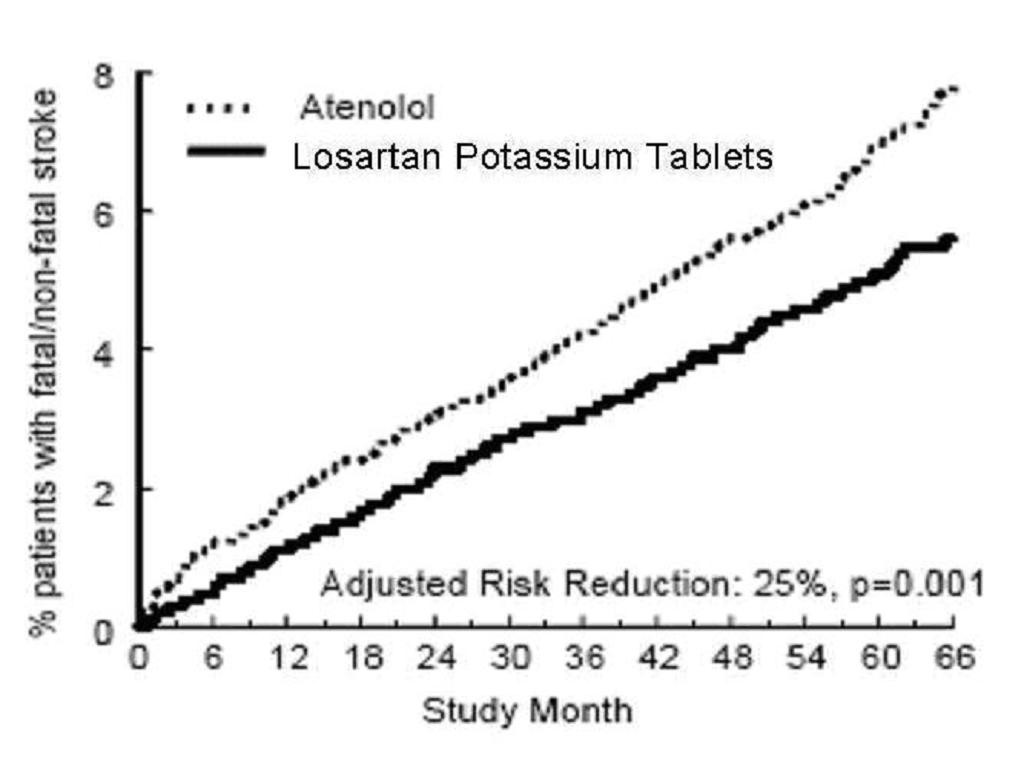
Figure 2. Kaplan-Meier estimates of the time to fatal/nonfatal stroke in the groups treated with losartan potassium tablets and atenolol. The Risk Reduction is adjusted for baseline Framingham risk score and level of electrocardiographic left ventricular hypertrophy.
Table 2 shows the results for the primary composite endpoint and the individual endpoints. The primary endpoint was the first occurrence of stroke, myocardial infarction or cardiovascular death, analyzed using an intention-to-treat (ITT) approach. The table shows the number of events for each component in two different ways. The Components of Primary Endpoint (as a first event) counts only the events that define the primary endpoint, while the Secondary Endpoints count all first events of a particular type, whether or not they were preceded by a different type of event.
Table 2 Incidence of Primary Endpoint Events
* Rate per 1000 patient-years of follow-upfor baseline Framingham risk score and level of electrocardiogram left ventricular hypertrophyFirst report of an event, in some cases the patient died subsequently to the event reportedDeath due to heart failure, non-coronary vascular disease, pulmonary embolism, or a cardiovascular cause other than stroke or coronary heart diseaseLosartan Potassium TabletsAtenololRisk Reduction95 % CIp-ValueN (%)Rate*N (%)Rate*Primary Composite Endpoint508 (11)23.8588 (13)27.913%2% to 23%0.021Components of Primary Composite Endpoint (as a first event)Stroke (nonfatal209 (5)286 (6)Myocardial infarction (nonfatal174 (4)168 (4)Cardiovascular mortality125 (3)134 (3)Secondary Endpoints (any time in study)Stroke (fatal/nonfatal)232 (5)10.8309 (7)14.525%11% to 37%0.001Myocardial infarction (fatal/nonfatal)198 (4)9.2188 (4)8.7-7%-13% to 12%0.491Cardiovascular mortality204 (4)9.2234 (5)10.611%-7% to 27%0.206Due to CHD125 (3)5.6124(3)5.6-3%-32% to 20%0.839Due to Stroke40 (1)1.862 (1)2.835%4% to 67%0.032Other39 (1)1.848 (1)2.216%-28% to 45%0.411Although the LIFE study favored losartan potassium tablets over atenolol with respect to the primary endpoint (p=0.021), this result is from a single study and, therefore, is less compelling than the difference between losartan potassium tablets and placebo. Although not measured directly, the difference between losartan potassium tablets and placebo is compelling because there is evidence that atenolol is itself effective (vs. placebo) in reducing cardiovascular events, including stroke, in hypertensive patients.
Other clinical endpoints of the LIFE study were: total mortality, hospitalization for heart failure or angina pectoris, coronary or peripheral revascularization procedures, and resuscitated cardiac arrest. There were no significant differences in the rates of these endpoints between the losartan potassium tablets and atenolol groups.
For the primary endpoint and stroke, the effects of losartan potassium tablets in patient subgroups defined by age, gender, race and presence or absence of isolated systolic hypertension (ISH), diabetes, and history of cardiovascular disease (CVD) are shown in Figure 3 below. Subgroup analyses can be difficult to interpret and it is not known whether these represent true differences or chance effects.

Symbols are proportional to sample size.
#Other includes Asian, Hispanic, Asiatic, Multi-race, Indian, Native American, European.
for baseline Framingham risk score and level of electrocardiographic left ventricular hypertophy.
Race: In the LIFE study, Black patients treated with atenolol were at lower risk of experiencing the primary composite endpoint compared with Black patients treated with losartan potassium tablets. In the subgroup of Black patients (n=533; 6% of the LIFE study patients), there were 29 primary endpoints among 263 patients on atenolol (11%, 26 per 1000 patient-years) and 46 primary endpoints among 270 patients (17%, 42 per 1000 patient-years) on losartan potassium tablets. This finding could not be explained on the basis of differences in the populations other than race or on any imbalances between treatment groups. In addition, blood pressure reductions in both treatment groups were consistent between Black and non-Black patients. Given the difficulty in interpreting subset differences in large trials, it cannot be known whether the observed difference is the result of chance. However, the LIFE study provides no evidence that the benefits of losartan potassium tablets on reducing the risk of cardiovascular events in hypertensive patients with left ventricular hypertrophy apply to Black patients.
Nephropathy in Type 2 Diabetic Patients: The Reduction of Endpoints in NIDDM with the Angiotensin II Receptor Antagonist Losartan (RENAAL) study was a randomized, placebo-controlled, double-blind, multicenter study conducted worldwide in 1513 patients with type 2 diabetes with nephropathy (defined as serum creatinine 1.3 to 3.0 mg/dl in females or maleskg and 1.5 to 3.0 mg/dl in males >60 kg and proteinuria [urinary albumin to creatinine ratiomg/g]).
Patients were randomized to receive losartan potassium tablets 50 mg once daily or placebo on a background of conventional antihypertensive therapy excluding ACE inhibitors and angiotensin II antagonists. After one month, investigators were instructed to titrate study drug to 100 mg once daily if the trough blood pressure goal (140/90 mmHg) was not achieved. Overall, 72% of patients received the 100-mg daily dose more than 50% of the time they were on study drug. Because the study was designed to achieve equal blood pressure control in both groups, other antihypertensive agents (diuretics, calcium-channel blockers, alpha- or beta-blockers, and centrally acting agents) could be added as needed in both groups. Patients were followed for a mean duration of 3.4 years.
The study population was diverse with regard to race (Asian 16.7%, Black 15.2%, Hispanic 18.3%, White 48.6%). Overall, 63.2% of the patients were men, and 66.4% were under the age of 65 years. Almost all of the patients (96.6%) had a history of hypertension, and the patients entered the trial with a mean serum creatinine of 1.9 mg/dl and mean proteinuria (urinary albumin/creatinine) of 1808 mg/g at baseline.
The primary endpoint of the study was the time to first occurrence of any one of the following events: doubling of serum creatinine, end-stage renal disease (ESRD) (need for dialysis or transplantation), or death. Treatment with losartan potassium tablets resulted in a 16% risk reduction in this endpoint (see Figure 4 and Table 3). Treatment with losartan potassium tablets also reduced the occurrence of sustained doubling of serum creatinine by 25% and ESRD by 29% as separate endpoints, but had no effect on overall mortality (see Table 3).
The mean baseline blood pressures were 152/82 mmHg for losartan potassium tablets plus conventional antihypertensive therapy and 153/82 mmHg for placebo plus conventional antihypertensive therapy. At the end of the study, the mean blood pressures were 143/76 mmHg for the group treated with losartan potassium tablets and 146/77 mmHg for the group treated with placebo.

Figure 4. Kaplan-Meier curve for the primary composite endpoint of doubling of serum creatinine, end stage renal disease (need for dialysis or transplantation) or death.
Table 3 Incidence of Primary Endpoint Events
IncidenceRisk Reduction95 % C.l.p-ValueLosartanPlaceboPrimary Composite Endpoint43.5%47.1%16.1%2.3% to 27.9%0.022Doubling of Serum Creatinine, ESRD and Death Occurring as a First EventDoubling of Serum Creatinine21.6%26.0%ESRD8.5%8.5%Death13.4%12.6%Overall Incidence of Doubling of Serum Creatinine, ESRD and DeathDoubling of Serum Creatinine21.6%26.0%25.3%7.8% to 39.4%0.006ESRD19.6%25.5%28.6%11.5% to 42.4%0.002Death21.0%20.3%-1.7%-26.9% to 18.6%0.884The secondary endpoints of the study were change in proteinuria, change in the rate of progression of renal disease, and the composite of morbidity and mortality from cardiovascular causes (hospitalization for heart failure, myocardial infarction, revascularization, stroke, hospitalization for unstable angina, or cardiovascular death). Compared with placebo, losartan potassium tablets significantly reduced proteinuria by an average of 34%, an effect that was evident within 3 months of starting therapy, and significantly reduced the rate of decline in glomerular filtration rate during the study by 13%, as measured by the reciprocal of the serum creatinine concentration. There was no significant difference in the incidence of the composite endpoint of cardiovascular morbidity and mortality.
The favorable effects of losartan potassium tablets were seen in patients also taking other anti-hypertensive medications (angiotensin II receptor antagonists and angiotensin converting enzyme inhibitors were not allowed), oral hypoglycemic agents and lipid-lowering agents.
For the primary endpoint and ESRD, the effects of losartan potassium tablets in patient subgroups defined by age, gender and race are shown in Table 4 below. Subgroup analyses can be difficult to interpret and it is not known whether these represent true differences or chance effects.
Table 4 Efficacy Outcomes within Demographic Subgroups
Primary Composite EndpointESRDNo. of PatientsLosartan Potassium Tablets Event Rate %Placebo Event Rate %Hazard Ratio (95% CI)Losartan Potassium Tablets Event Rate %Placebo Event Rate %Hazard Ratio (95% CI)Overall Results151343.547.10.839 (0.721, 0.977)19.625.50.714 (0.576, 0.885)Age<65 years100544.149.00.784 (0.653, 0.941)21.128.50.670 (0.521, 0.863)years50842.343.50.978 (0.749, 1.277)16.519.60.847 (0.560, 1.281)GenderFemale55747.854.10.762 (0.603, 0.962)22.832.80.601 (0.436, 0.828)Male95640.943.30.892 (0.733, 1.085)17.521.50.809 (0.605, 1.081)RaceAsian25241.954.80.655 (0.453, 0.947)18.827.40.625 (0.367, 1.066)Black23040.039.00.983 (0.647, 1.495)17.621.00.831 (0.456, 1.516)Hispanic27755.054.01.003 (0.728, 1.380)30.028.51.024 (0.661, 1.586)White73540.543.20.809 (0.645, 1.013)16.223.90.596 (0.427, 0.831)
INDICATIONS & USAGE
PRECAUTIONSRaceCLINICAL PHARMACOLOGYPharmacodynamics and Clinical EffectsReduction in the Risk of StrokeRace
CLINICAL PHARMACOLOGYPharmacodynamics and Clinical Effects
LOSARTAN POTASSIUM CONTRAINDICATIONS
WARNINGS
DOSAGE AND ADMINISTRATION
PRECAUTIONS
ADVERSE REACTIONSPost-Marketing Experience
DOSAGE AND ADMINISTRATIONCLINICAL PHARMACOLOGYPharmacokinetics
ADVERSE REACTIONS
INFORMATION FOR PATIENTS
PRECAUTIONSDrug Interactions
DRUG INTERACTIONS
CLINICAL PHARMACOLOGYDrug InteractionsCARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
WARNINGSFetal/Neonatal Morbidity and MortalityNURSING MOTHERS
PEDIATRIC USE
CLINICAL PHARMACOLOGYPharmacokineticsSpecial PopulationsPharmacodynamics and Clinical EffectsDOSAGE AND ADMINISTRATIONGERIATRIC USE
CLINICAL PHARMACOLOGY Pharmacodynamics and Clinical EffectsReduction in the Risk of Stroke).
LOSARTAN POTASSIUM ADVERSE REACTIONS
PRECAUTIONS
OVERDOSAGE
DOSAGE & ADMINISTRATION
WARNINGSHypotensionVolume-Depleted PatientsPRECAUTIONSGeneral
CLINICAL PHARMACOLOGYPharmacodynamics and Clinical Effects
CLINICAL PHARMACOLOGYPharmacodynamics and Clinical Effects
CLINICAL PHARMACOLOGYPharmacokineticsSpecial PopulationsPharmacodynamics and Clinical EffectsWARNINGSHypotensionVolume-Depleted Patients
CLINICAL PHARMACOLOGYPharmacokineticsSpecial PopulationsPharmacodynamics and Clinical EffectsPRECAUTIONS
CLINICAL PHARMACOLOGYPharmacodynamics and Clinical EffectsReduction in the Risk of Stroke
CLINICAL PHARMACOLOGYPharmacodynamics and Clinical Effects
HOW SUPPLIED
STORAGE AND HANDLING
INFORMATION FOR PATIENTS
Losartan Potassium Tablets, USP25mg, 50mg, 100mg
Rx only
What is the most important information I should know about losartan potassium tablets?
Do not take losartan potassium tablets if you are pregnant or plan to become pregnant. Losartan potassium tablets can harm your unborn baby causing injury and even death. Stop taking losartan potassium tablets if you become pregnant and call your doctor right away.
What are losartan potassium tablets?
-
● alone or with other blood pressure medicines to lower high blood pressure (hypertension).
-
● to lower the chance of stroke in patients with high blood pressure and a heart problem called left ventricular hypertrophy. Losartan potassium tablets may not help Black patients with this problem.
-
● to slow the worsening of diabetic kidney disease (nephropathy) in patients with type 2 diabetes who have or had high blood pressure.
High Blood Pressure (hypertension).
Left Ventricular Hypertrophy (LVH)
Type 2 Diabetes with Nephropathy.
Who should not take losartan potassium tablets?
-
● Do not take losartan potassium tablets if you are allergic to any of the ingredients in losartan potassium tablets. See the end of this leaflet for a complete list of ingredients in losartan potassium tablets.
-
● are pregnant or planning to become pregnant.See "What is the most important information I should know about losartan potassium tablets?"
-
● are breast-feeding.It is not known if losartan potassium tablets passes into your breast milk. You should choose either to take losartan potassium tablets or breast-feed, but not both.
-
● are vomiting a lot or having a lot of diarrhea
-
● have liver problems
-
● have kidney problems
-
● potassium supplements
-
● salt substitutes containing potassium
-
● water pills (diuretics)
-
● medicines used to treat pain and arthritis, called non-steroidal anti-inflammatory drugs (NSAIDs), including COX-2 inhibitors
-
● Take losartan potassium tablets exactly as prescribed by your doctor. Your doctor may change your dose if needed.
-
● Losartan potassium tablets can be taken with or without food.
-
● If you miss a dose, take it as soon as you remember. If it is close to your next dose, do not take the missed dose. Just take the next dose at your regular time.
-
● If you take too much losartan potassium tablets, call your doctor or Poison Control Center, or go to the nearest hospital emergency room right away.
-
● Injury or death of unborn babies. See "What is the most important information I should know about losartan potassium tablets?"
-
● Allergic reaction.Symptoms of an allergic reaction are swelling of the face, lips, throat or tongue. Get emergency medical help right away and stop taking losartan potassium tablets.
-
● Low blood pressure (hypotension).Low blood pressure may cause you to feel faint or dizzy. Lie down if you feel faint or dizzy. Call your doctor right away.
-
● For people who already have kidney problems, you may see a worsening in how well your kidneys work.Call your doctor if you get swelling in your feet, ankles, or hands, or unexplained weight gain.
-
● "colds" (upper respiratory infection)
-
● dizziness
-
● stuffy nose
-
● back pain
-
● diarrhea
-
● tiredness
-
● low blood sugar
-
● chest pain
-
● high blood potassium
-
● low blood pressure
How do I store losartan potassium tablets?
-
● Store losartan potassium tablets at 59to 86(15to 30
-
● Keep losartan potassium tablets in a tightly closed container that protects the medicine from light.
-
● Keep losartan potassium tablets and all medicines out of the reach of children.
What are the ingredients in losartan potassium tablets?
Active ingredients:
Inactive ingredients:
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION


Losartan PotassiumLosartan Potassium TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!