Lovastatin
FULL PRESCRIBING INFORMATION: CONTENTS*
- LOVASTATIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- LOVASTATIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- LOVASTATIN ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
LOVASTATIN DESCRIPTION
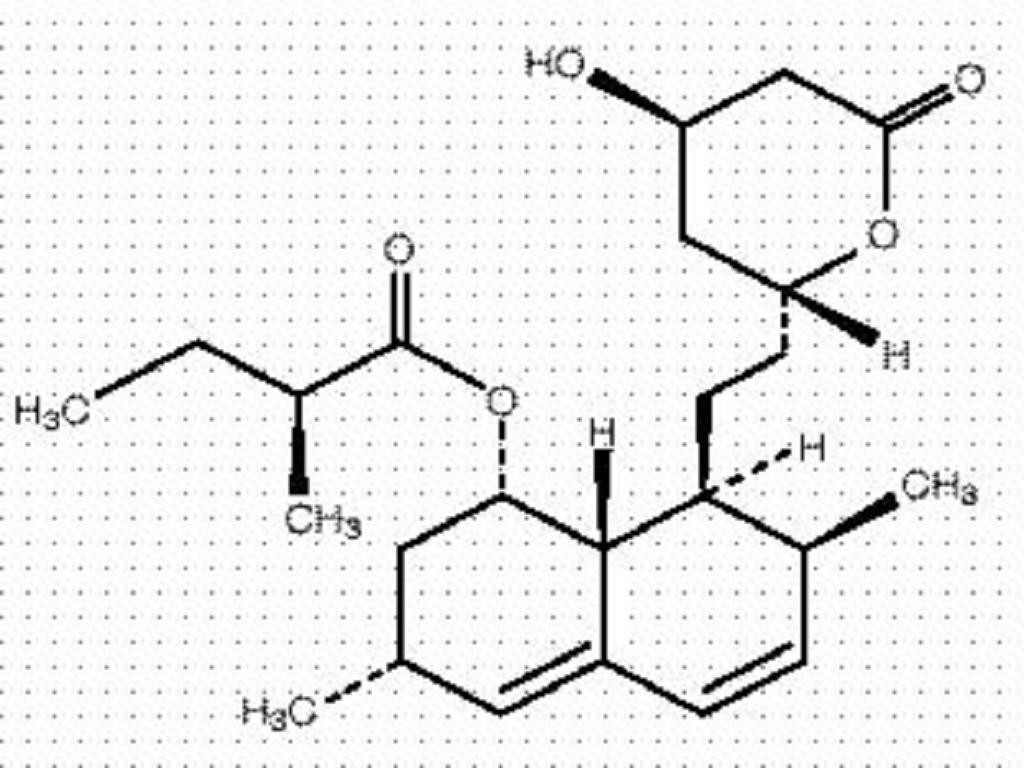
CLINICAL PHARMACOLOGY
Clinical Studies
PRECAUTIONS, Geriatric Use
WARNINGS, Myopathy/RhabdomyolsisPRECAUTIONS, Drug Interactions
PRECAUTIONS, Drug Interactions
Clinical Studies In Adolescent Patients:
INDICATIONS & USAGE
CLINICAL PHARMACOLOGY, Clinical Studies
General Recommendations:
Lipid Elevations
LOVASTATIN CONTRAINDICATIONS
WARNINGS
PRECAUTIONS, PregnancyNursing MothersLovastatin should be administered to women of childbearing age only when such patients are highly unlikely to conceive.PRECAUTIONS, Pregnancy
WARNINGS
Myopathy/Rhabdomyolysis:As with other HMG-CoA reductase inhibitors, the risk of myopathy/rhabdomyolysis is dose related.
All patients starting therapy with lovastatin, or whose dose of lovastatin is being increased, should be advised of the risk of myopathy and told to report promptly any unexplained muscle pain, tenderness or weakness. Lovastatin therapy should be discontinued immediately if myopathy is diagnosed or suspected.
The risk of myopathy/rhabdomyolysis is increased by concomitant use of lovastatin with the following:
Potent Inhibitors Of CYP3A4:
The use of lovastatin concomitantly with the potent CYP3A4 inhibitors itraconazole, ketoconazole, erythromycin, clarithromycin, telithromycin, HIV protease inhibitors, nefazodone, or large quantities of grapefruit juice (>1 quart daily) should be avoided.
Gemfibrozil, Particularly With Higher Doses Of Lovastatin:The dose of lovastatin should not exceed 20 mg daily in patients receiving concomitant medication with gemfibrozil. The combined use of lovastatin with gemfibrozil should be avoided, unless the benefits are likely to outweigh the increased risks of this drug combination.
Other Lipid-lowering Drugs (Other Fibrates Or1 g/day Of Niacin):The dose of lovastatin should not exceed 20 mg daily in patients receiving concomitant medication with other fibrates or1 g/day of niacin.The benefit of further alterations in lipid levels by the combined use of lovastatin with other fibrates or niacin should be carefully weighed against the potential risks of these combinations.
Cyclosporine Or Danazol, With Higher Doses Of Lovastatin:The dose of lovastatin should not exceed 20 mg daily in patients receiving concomitant medication with cyclosporine or danazol.
Amiodarone Or Verapamil:The dose of lovastatin should not exceed 40 mg daily in patients receiving concomitant medication with amiodarone or verapamil.
CLINICAL PHARMACOLOGY, PharmacokineticsPRECAUTIONS, Drug InteractionsDOSAGE AND ADMINISTRATION).
Table VIDrug Interactions Associated with Increased Risk of Myopathy/Rhabdomyolysis
Interacting AgentsPrescribing RecommendationsItraconazoleAvoid lovastatinKetoconazoleErythromycinClarithromycinTelithromycinHIV protease inhibitorsNefazodoneGemfibrozilDo not exceed 20 mg lovastatinOther fibratesLipid-lowering doses (g/day)of niacinCyclosporineDanazolAmiodaroneDo not exceed 40 mg lovastatin dailyVerapamilGrapefruit juiceAvoid large quantities of grapefruit juice (>1 quart daily)Liver Dysfunction:Persistent increases (to more than 3 times the upper limit of normal) in serum transaminases occurred in 1.9% of adult patients who received lovastatin for at least one year in early clinical trials(seeADVERSE REACTIONS). When the drug was interrupted or discontinued in these patients, the transaminase levels usually fell slowly to pretreatment levels. The increases usually appeared 3 to 12 months after the start of therapy with lovastatin, and were not associated with jaundice or other clinical signs or symptoms. There was no evidence of hypersensitivity. In the EXCEL study (seeCLINICAL PHARMACOLOGY, Clinical Studies), the incidence of persistent increases in serum transaminases over 48 weeks was 0.1% for placebo, 0.1% at 20 mg/day, 0.9% at 40 mg/day, and 1.5% at 80 mg/day in patients on lovastatin. However, in post-marketing experience with lovastatin, symptomatic liver disease has been reported rarely at all dosages (seeADVERSE REACTIONS).
In AFCAPS/TexCAPS, the number of participants with consecutive elevations of either alanine aminotransferase (ALT) or aspartate aminotransferase (AST) (> 3 times the upper limit of normal), over a median of 5.1 years of follow-up, was not significantly different between the lovastatin and placebo groups (18 [0.6%] vs. 11 [0.3%]). The starting dose of lovastatin was 20 mg/day; 50% of the lovastatin treated participants were titrated to 40 mg/day at Week 18. Of the 18 participants on lovastatin with consecutive elevations of either ALT or AST, 11 (0.7%) elevations occurred in participants taking 20 mg/day, while 7 (0.4%) elevations occurred in participants titrated to 40 mg/day. Elevated transaminases resulted in discontinuation of 6 (0.2%) participants from therapy in the lovastatin group (n=3,304) and 4 (0.1%) in the placebo group (n=3,301).
It is recommended that liver function tests be performed prior to initiation of therapy in patients with a history of liver disease, or when otherwise clinically indicated. It is recommended that liver function tests be performed in all patients prior to use of 40 mg or more daily and thereafter when clinically indicated. Patients who develop increased transaminase levels should be monitored with a second liver function evaluation to confirm the finding and be followed thereafter with frequent liver function tests until the abnormality(ies) returns to normal. Should an increase in AST or ALT of three times the upper limit of normal or greater persist, withdrawl of therapy with lovastatin is recommended.
The drug should be used with caution in patients who consume substantial quantities of alcohol and/or have a past history of liver disease. Active liver disease or unexplained transaminase elevations are contraindications to the use of lovastatin.
As with other lipid-lowering agents, moderate (less than three times the upper limit of normal) elevations of serum transaminases have been reported following therapy with lovastatin (seeADVERSE REACTIONS). These changes appeared soon after initiation of therapy with lovastatin, were often transient, were not accompanied by any symptoms and interruption of treatment was not required.
PRECAUTIONS
GeneralWARNINGSADVERSE REACTIONS
ADVERSE REACTIONS
INFORMATION FOR PATIENTS
Patients should be advised about substances they should not take concomitantly with lovastatin and be advised to report promptly unexplained muscle pain, tenderness, or weakness (see list below and WARNINGS, Myopathy/Rhabdomyolysis). Patients should also be advised to inform other physicians prescribing a new medication that they are taking lovastatin.DRUG INTERACTIONS
WARNINGS, Myopathy/Rhabdomyolysis,CLINICAL PHARMACOLOGY, Pharmacokinetics
Itraconazole
Ketoconazole
Erythromycin
Clarithromycin
Telithromycin
HIV protease inhibitors
Nefazodone
Large quantities of grapefruit juice (>1 quart daily)
WARNINGS, Myopathy/Rhabdomyolysis
Other fibrates
Niacin (nicotinic acid) (1 g/day)
Other Drug Interactions
WARNINGS, Myopathy/RhabdomyolysisCLINICAL PHARMACOLOGY, Pharmacokinetics).
Amiodarone or verapamil: The risk of myopathy/rhabdomyolysis is increased when either amiodarone or verapamil is used concomitantly with a closely related member of the HMG-CoA reductase inhibitor class (seeWARNINGS, Myopathy/Rhabdomyolysis).
Coumarin anticoagulants: In a small clinical trial in which lovastatin was administered to warfarin treated patients, no effect on prothrombin time was detected. However, another HMG-CoA reductase inhibitor has been found to produce a less than two-second increase in prothrombin time in healthy volunteers receiving low doses of warfarin. Also, bleeding and/or increased prothrombin time have been reported in a few patients taking coumarin anticoagulants concomitantly with lovastatin. It is recommended that in patients taking anticoagulants, prothrombin time be determined before starting lovastatin and frequently enough during early therapy to insure that no significant alteration of prothrombin time occurs. Once a stable prothrombin time has been documented, prothrombin times can be monitored at the intervals usually recommended for patients on coumarin anticoagulants. If the dose of lovastatin is changed, the same procedure should be repeated. Lovastatin therapy has not been associated with bleeding or with changes in prothrombin time in patients not taking anticoagulants.
Propranolol: In normal volunteers, there was no clinically significant pharmacokinetic or pharmacodynamic interaction with concomitant administration of single doses of lovastatin and propranolol.
Digoxin: In patients with hypercholesterolemia, concomitant administration of lovastatin and digoxin resulted in no effect on digoxin plasma concentrations.
Oral hypoglycemic agents: In pharmacokinetic studies of lovastatin in hypercholesterolemic noninsulin dependent diabetic patients, there was no drug interaction with glipizide or with chlorpropamide (seeCLINICAL PHARMACOLOGY, Clinical Studies).
Endocrine Function:HMG-CoA reductase inhibitors interfere with cholesterol synthesis and as such might theoretically blunt adrenal and/or gonadal steroid production. Results of clinical trials with drugs in this class have been inconsistent with regard to drug effects on basal and reserve steroid levels. However, clinical studies have shown that lovastatin does not reduce basal plasma cortisol concentration or impair adrenal reserve, and does not reduce basal plasma testosterone concentration. Another HMG-CoA reductase inhibitor has been shown to reduce the plasma testosterone response to HCG. In the same study, the mean testosterone response to HCG was slightly but not significantly reduced after treatment with lovastatin 40 mg daily for 16 weeks in 21 men. The effects of HMG-CoA reductase inhibitors on male fertility have not been studied in adequate numbers of male patients. The effects, if any, on the pituitary-gonadal axis in pre-menopausal women are unknown. Patients treated with lovastatin who develop clinical evidence of endocrine dysfunction should be evaluated appropriately. Caution should also be exercised if an HMG-CoA reductase inhibitor or other agent used to lower cholesterol levels is administered to patients also receiving other drugs (e.g., ketoconazole, spironolactone, cimetidine) that may decrease the levels or activity of endogenous steroid hormones.
CNS Toxicity:Lovastatin produced optic nerve degeneration (Wallerian degeneration of retinogeniculate fibers) in clinically normal dogs in a dose-dependent fashion starting at 60 mg/kg/day, a dose that produced mean plasma drug levels about 30 times higher than the mean drug level in humans taking the highest recommended dose (as measured by total enzyme inhibitory activity). Vestibulocochlear Wallerian-like degeneration and retinal ganglion cell chromatolysis were also seen in dogs treated for 14 weeks at 180 mg/kg/day, a dose which resulted in a mean plasma drug level (Cmax) similar to that seen with the 60 mg/kg/day dose.
CNS vascular lesions, characterized by perivascular hemorrhage and edema, mononuclear cell infiltration of perivascular spaces, perivascular fibrin deposits and necrosis of small vessels, were seen in dogs treated with lovastatin at a dose of 180 mg/kg/day, a dose which produced plasma drug levels (Cmax) which were about 30 times higher than the mean values in humans taking 80 mg/day.
Similar optic nerve and CNS vascular lesions have been observed with other drugs of this class.
Cataracts were seen in dogs treated for 11 and 28 weeks at 180 mg/kg/day and 1 year at 60 mg/kg/day.
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
CONTRAINDICATIONSCONTRAINDICATIONS
NURSING MOTHERS
CONTRAINDICATIONSPEDIATRIC USE
Doses greater than 40 mg have not been studied in this population.CLINICAL PHARMACOLOGY, Clinical Studies in Adolescent PatientsADVERSE REACTIONS, Adolescent PatientsDOSAGE AND ADMINISTRATION, Adolescent Patients (10-17 years of age) with Heterozygous Familial Hypercholesterolemia. Adolescent femalesshould be counseled on appropriate contraceptive methods while on lovastatin therapy (seeCONTRAINDICATIONSandPRECAUTIONS, Pregnancy).Lovastatin has not been studied in pre-pubertal patients or patients younger than 10 years of age.GERIATRIC USE
CLINICAL PHARMACOLOGYLOVASTATIN ADVERSE REACTIONS
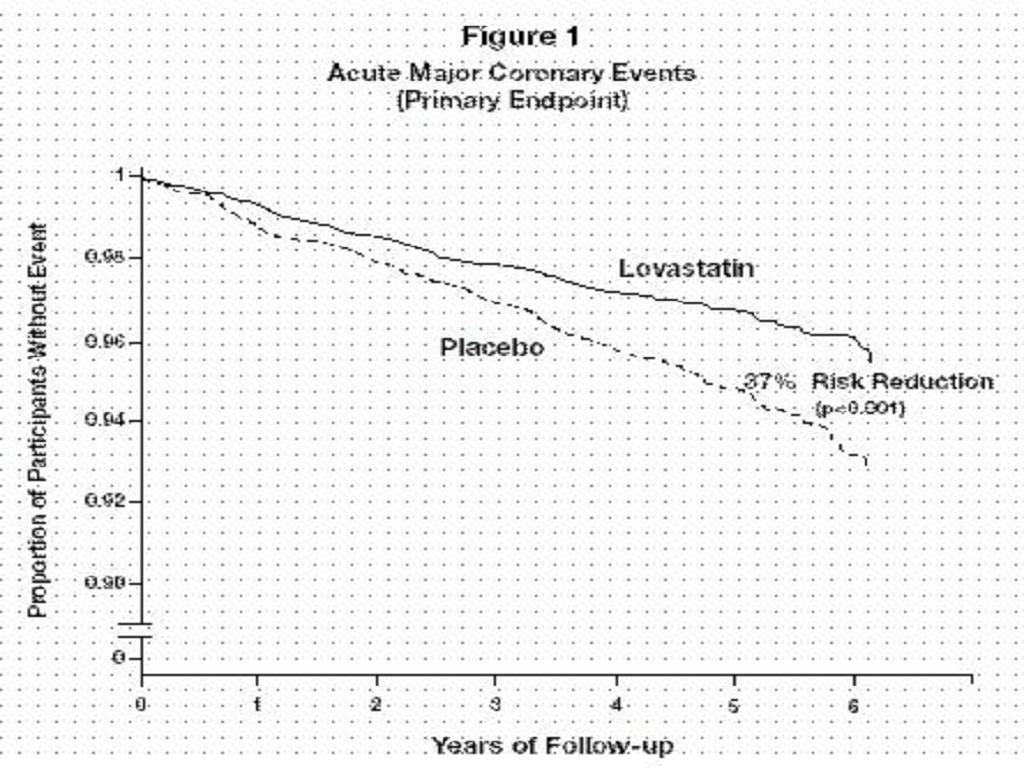
Expanded Clinical Evaluation of Lovastatin [EXCEL] Study
WARNINGS, Liver DysfunctionWARNINGS, Myopathy/Rhabdomyolysis
CLINICAL PHARMACOLOGY, Clinical Studies
CLINICAL PHARMACOLOGY, Clinical StudiesADVERSE REACTIONS, Expanded Clinical Evaluation of Lovastatin (EXCEL) Study
WARNINGS, Myopathy/Rhabdomyolysis
Adolescent Patients (Ages 10-17 Years):
CLINICAL PHARMACOLOGY, Clinical Studies in Adolescent PatientsPRECAUTIONS, Pediatric Use
OVERDOSAGE
DOSAGE & ADMINISTRATION
CLINICAL PHARMACOLOGYINDICATIONS AND USAGE
Dosage In Patients Taking Cyclosporine Or Danazol:
WARNINGS, Myopathy/Rhabdomyolysis
Dosage In Patients Taking Amiodarone Or Verapamil:
WARNINGS, Myopathy/RhabdomyolysisPRECAUTIONS, Drug Interactions, Other Drug Interactions
Adolescent Patients (10-17 Years Of Age) With Heterozygous Familial Hypercholesterolemia:
CLINICAL PHARMACOLOGYINDICATIONS AND USAGE
Concomitant Lipid-Lowering Therapy:
WARNINGS, Myopathy/RhabdomyolysisPRECAUTIONS, Drug Interactions
Dosage In Patients With Renal Insufficiency:
CLINICAL PHARMACOLOGYWARNINGS, Myopathy/Rhabdomyolysis
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

LovastatinLovastatin TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!