Lovastatin
FULL PRESCRIBING INFORMATION: CONTENTS*
- LOVASTATIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- LOVASTATIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- LOVASTATIN ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
LOVASTATIN DESCRIPTION
CLINICAL PHARMACOLOGY
Tables I-IIIunderClinical Studies). The effects of lovastatin on Lp (a), fibrinogen, and certain other independent biochemical risk markers for coronary heart disease are unknown.
Pharmacokinetics
PRECAUTIONS,Geriatric Use).
WARNINGS,Myopathy/RhabdomyolysisandPRECAUTIONS,Drug Interactions).
PRECAUTIONS,Drug Interactions.) Grapefruit juice contains one or more components that inhibit CYP3A4 and can increase the plasma concentrations of drugs metabolized by CYP3A4. In one study **, 10 subjects consumed 200 mL of double-strength grapefruit juice (one can of frozen concentrate diluted with one rather than 3 cans of water) three times daily for 2 days and an additional 200 mL double-strength grapefruit juice together with and 30 and 90 minutes following a single dose of 80 mg lovastatin on the third day. This regimen of grapefruit juice resulted in a mean increase in the serum concentration of lovastatin and itsmetabolite (as measured by the area under the concentration-time curve) of 15-fold and 5-fold, respectively [as measured using a chemical assay--high performance liquid chromatography.] In a second study, 15 subjects consumed one 8 oz glass of single-strength grapefruit juice (one can of frozen concentrate diluted with 3 cans of water) with breakfast for 3 consecutive days and a single dose of 40 mg lovastatin in the evening of the third day. This regimen of grapefruit juice resulted in a mean increase in the plasma concentration (as measured by the area under the concentration-time curve) of active and total HMG-CoA reductase inhibitory activity [using an enzyme inhibition assay both before (for active inhibitors) and after (for total inhibitors) base hydrolysis] of 1.34-fold and 1.36-fold, respectively, and of lovastatin and itsmetabolite [measured using a chemical assay--liquid chromatography/tandem mass spectrometry--different from that used in the first ** study] of 1.94-fold and 1.57-fold, respectively. The effect of amounts of grapefruit juice between those used in these two studies on lovastatin pharmacokinetics has not been studied.
Clinical Studies in Adults
Tables IthroughIIIfor dose response results).
Table I.
TABLE I Lovastatin vs Placebo (Mean Percent Change from Baseline After 6 Weeks)
Table II.
TABLE II Lovastatin vs. Cholestyramine (Percent Change from Baseline After 12 Weeks)
Expanded Clinical Evaluation of Lovastatin (EXCEL) Study
Table III) in lovastatin treated patients were dose-related and significantly different from placebo (pThese results were sustained throughout the study.
TABLE III Lovastatin vs. Placebo (Percent Change from Baseline-- Average Values Between Weeks 12 and 48)
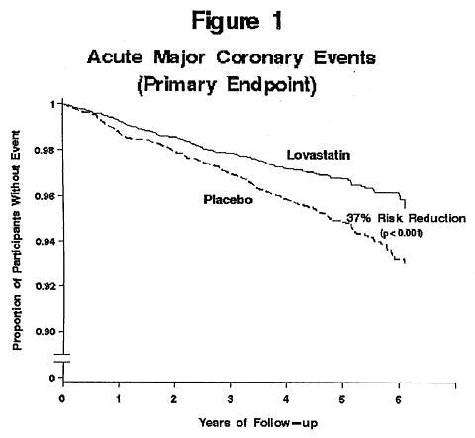
Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS)
Figure 1risk factors had risk reductions (RR) in both acute major coronary events (RR 43%) and coronary revascularization procedures (RR 37%). Because there were too few events among those participants with age as their only risk factor in this study, the effect of lovastatin on outcomes could not be adequately assessed in this subgroup.
Atherosclerosis
Eye
Clinical Studies in Adolescent Patients
Table IV).
TABLE IV Lipid-lowering Effects of Lovastatin in Adolescent Boys with Heterozygous Familial Hypercholesterolemia (Mean Percent Change from Baseline at week 48 in Intention-to-Treat Population)
Table V).
TABLE V Lipid-lowering Effects of Lovastatin in Post-menarchal Girls with Heterozygous Familial Hypercholesterolemia (Mean Percent Change from Baseline at Week 24 in Intention-to-Treat Population)
INDICATIONS & USAGE
CLINICAL PHARMACOLOGY, Clinical Studies.)
Hypercholesterolemia
Adolescent Patients with Heterozygous Familial Hypercholesterolemia
General Recommendations
***Classification of Hyperlipoproteinemias
NCEP Treatment Guidelines: LDL-C Goals and Cutpoints for Therapeutic Lifestyle Changes and Drug Therapy in Different Risk Categories
LOVASTATIN CONTRAINDICATIONS
WARNINGS).
Lovastatin should be administered to women of childbearing age only when such patients are highly unlikely to conceive.If the patient becomes pregnant while taking this drug, lovastatin should be discontinued immediately and the patient should be apprised of the potential hazard to the fetus (seePRECAUTIONS,Pregnancy).
WARNINGS
Myopathy/RhabdomyolysisAs with other HMG-CoA reductase inhibitors, the risk of myopathy/rhabdomyolysis is dose related.In a clinical study (EXCEL) in which patients were carefully monitored and some interacting drugs were excluded, there was one case of myopathy among 4933 patients randomized to lovastatin 20-40 mg daily for 48 weeks, and 4 among 1649 patients randomized to 80 mg daily.
All patients starting therapy with lovastatin, or whose dose of lovastatin is being increased, should be advised of the risk of myopathy and told to report promptly any unexplained muscle pain, tenderness or weakness. Lovastatin therapy should be discontinued immediately if myopathy is diagnosed or suspected.In most cases, musclesymptoms and CK increases resolved when treatment was promptly discontinued. Periodic CK mdeterminations may be considered in patients starting therapy with lovastatin or whose dose is being increased, but there is no assurance that such monitoring will prevent myopathy.
The risk of myopathy/rhabdomyolysis is increased by concomitant use of lovastatin with the following:
Potent inhibitors of CYP3A4:Lovastatin, like several other inhibitors of HMG-CoA reductase, is a substrate of cytochrome P450 3A4 (CYP3A4). When lovastatin is used with a potent inhibitor of CYP3A4, elevated plasma levels of HMG-CoA reductase inhibitory activity can increase the risk of myopathy and rhabdomyolysis, particularly with higher doses of lovastatin.
The use of lovastatin concomitantly with the potent CYP3A4 inhibitors itraconazole, ketoconazole, erythromycin, clarithromycin, telithromycin, HIV protease inhibitors, nefazodone, or large quantities of grapefruit juice (>1 quart daily) should be avoided.
Gemfibrozil, particularly with higher doses of lovastatin:The dose of lovastatin should not exceed 20 mg daily in patients receiving concomitant medication with gemfibrozil. The combined use of lovastatin with gemfibrozil should be avoided, unless the benefits are likely to outweigh the increased risks of this drug combination.
Other lipid-lowering drugs (other fibrates org/day of niacin):The dose of lovastatin should not exceed 20 mg daily in patients receiving concomitant medication with other fibrates org/day of niacin.Caution should be used when prescribing other fibrates or lipid-lowering doses (g/day) of niacin with lovastatin, as these agents can cause myopathy when given alone.The benefit of further alterations in lipid levels by the combined use of lovastatin with other fibrates or niacin should be carefully weighed against the potential risks of these combinations.
Cyclosporine or danazol, with higher doses of lovastatin:The dose of lovastatin should not exceed 20 mg daily in patients receiving concomitant medication with cyclosporine or danazol.The benefits of the use of lovastatin in patients receiving cyclosporine or danazol should be carefully weighed against the risks of these combinations.
Amiodarone or verapamil:The dose of lovastatin should not exceed 40 mg daily in patients receiving concomitant medication with amiodarone or verapamil.The combined use of lovastatin at doses higher than 40 mg daily with amiodarone or verapamil should be avoided unless the clinical benefit is likely to outweigh the increased risk of myopathy. The risk of myopathy/rhabdomyolysis is increased when either amiodarone or verapamil is used concomitantly with higher doses of a closely related member of the HMG-CoA reductase inhibitor class.
Table VI( (seealso CLINICAL PHARMACOLOGY, PharmacokineticsPRECAUTIONS, Drug InteractionsDOSAGE AND ADMINISTRATION).
Table VI Drug Interactions Associated with Increased Risk of Myopathy/Rhabdomyolysis
Liver Dysfunction
Persistent increases (to more than 3 times the upper limit of normal) in serum transaminases occurred in 1.9% of adult patients who received lovastatin for at least one year in early clinical trials(seeADVERSE REACTIONS). When the drug was interrupted or discontinued in these patients, the transaminase levels usually fell slowly to pretreatment levels. The increases usually appeared 3 to 12 months after the start of therapy with lovastatin, and were not associated with jaundice or other clinical signs or symptoms. There was no evidence of hypersensitivity. In the EXCEL study (seeCLINICAL PHARMACOLOGY,Clinical Studies), the incidence of persistent increases in serum transaminases over 48 weeks was 0.1% for placebo, 0.1% at 20 mg/day, 0.9% at 40 mg/day, and 1.5% at 80 mg/day in patients on lovastatin. However, in post-marketing experience with lovastatin, symptomatic liver disease has been reported rarely at all dosages (seeADVERSE REACTIONS).
ADVERSE REACTIONS). These changes appeared soon after initiation of therapy with lovastatin, were often transient, were not accompanied by any symptoms and interruption of treatment was not required.
PRECAUTIONS
GeneralWARNINGSandADVERSE REACTIONS). This should be considered in the differential diagnosis of chest pain in a patient on therapy with lovastatin.
Homozygous Familial Hypercholesterolemia
ADVERSE REACTIONS) in these homozygous patients.
Information for Patients
Patients should be advised about substances they should not take concomitantly with lovastatin and be advised to report promptly unexplained muscle pain, tenderness, or weakness (see list below and WARNINGS, Myopathy/Rhabdomyolysis). Patients should also be advised to inform other physicians prescribing a new medication that they are taking lovastatin.
Drug Interactions
See WARNINGS, Myopathy/Rhabdomyolysis, and CLINICAL PHARMACOLOGY, Pharmacokinetics.
Itraconazole
Ketoconazole
Erythromycin
Clarithromycin
Telithromycin
HIV protease inhibitors
Nefazodone
Cyclosporine
Large quantities of grapefruit juice (>1 quart daily)
See WARNINGS, Myopathy/Rhabdomyolysis.
Gemfibrozil
Other fibrates
Niacin (nicotinic acid) (g/day)
WARNING,Myopathy/Rhabdomyolysis).
WARNINGS,Myopathy/RhabdomyolysisCLINICAL PHARMACOLOGY,Pharmacokinetics).
CLINICAL PHARMACOLOGY,Clinical Studies).
Endocrine Function
CNS Toxicity
Carcinogenesis, Mutagenesis, Impairment of Fertility
Pregnancy
CONTRAINDICATIONS.
CONTRAINDICATIONS). Lovastatin should be administered to women of childbearing potential only when such patients are highly unlikely to conceive and have been informed of the potential hazards. Treatment should be immediately discontinued as soon as pregnancy is recognized.
Nursing Mothers
CONTRAINDICATIONS).
Pediatric Use
Doses greater than 40 mg have not been studied in this population.In these limited controlled studies, there was no detectable effect on growth or sexual maturation in the adolescent boys or on menstrual cycle length in girls. SeeCLINICAL PHARMACOLOGY,Clinical Studies in Adolescent PatientsADVERSE REACTIONS,Adolescent PatientsDOSAGE AND ADMINISTRATION,Adolescent Patients (10-17 years of age) with Heterozygous Familial Hypercholesterolemia. Adolescent females should be counseled on appropriate contraceptive methods while on lovastatin therapy (seeCONTRAINDICATIONSandPRECAUTIONS,Pregnancy).Lovastatin has not been studied in pre-pubertal patients or patients younger than 10 years of age.
Geriatric Use
CLINICAL PHARMACOLOGY).
LOVASTATIN ADVERSE REACTIONS
Phase III Clinical Studies
Expanded Clinical Evaluation of Lovastatin [EXCEL] Study).
WARNINGS,Liver Dysfunction). About 11% of patients had elevations of CK levels of at least twice the normal value on one or more occasions. The corresponding values for the control agent cholestyramine was 9 percent. This was attributable to the noncardiac fraction of CK. Large increases in CK have sometimes been reported (seeWARNINGS,Myopathy/Rhabdomyolysis).
Expanded Clinical Evaluation of Lovastatin (EXCEL) Study
CLINICAL PHARMACOLOGY,Clinical Studies), 4.6% of the patients treated up to 48 weeks were discontinued due to clinical or laboratory adverse experiences which were rated by the investigator as possibly, probably or definitely related to therapy with lovastatin. The value for the placebo group was 2.5%.
Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS)
CLINICAL PHARMACOLOGY,Clinical Studies) involving 6,605 participants treated with 20-40 mg/day of lovastatin (n=3,304) or placebo (n=3,301), the safety and tolerability profile of the group treated with lovastatin was comparable to that of the group treated with placebo during a median of 5.1 years of follow-up. The adverse experiences reported in AFCAPS/TexCAPS were similar to those reported in EXCEL (seeADVERSE REACTIONS,Expanded Clinical Evaluation of Lovastatin (EXCEL) Study).
Concomitant Therapy
WARNINGS,Myopathy/Rhabdomyolysis).
Adolescent Patients (ages 10-17 years)
CLINICAL PHARMACOLOGY,Clinical Studies in Adolescent PatientsandPRECAUTIONS,Pediatric Use).
OVERDOSAGE
DOSAGE & ADMINISTRATION
Adult Patients
CLINICAL PHARMACOLOGY). Patients requiring reductions in LDL-C of 20% or more to achieve their goal (seeINDICATIONS AND USAGE) should be started on 20 mg/day of lovastatin. A starting dose of 10 mg may be considered for patients requiring smaller reductions. Adjustments should be made at intervals of 4 weeks or more.
Dosage in Patients taking Cyclosporine or Danazol
WARNINGS,Myopathy/Rhabdomyolysis), therapy should begin with 10 mg of lovastatin and should not exceed 20 mg/day.
Dosage in Patients taking Amiodarone or Verapamil
WARNINGS,Myopathy/RhabdomyolysisandPRECAUTIONS,Drug Interactions,Other drug interactions).
Adolescent Patients (10-17 years of age) with Heterozygous Familial Hypercholesterolemia
CLINICAL PHARMACOLOGY, andINDICATIONS AND USAGE). Patients requiring reductions in LDL-C of 20% or more to achieve their goal should be started on 20 mg/day of lovastatin. A starting dose of 10 mg may be considered for patients requiring smaller reductions. Adjustments should be made at intervals of 4 weeks or more.
Concomitant Lipid-Lowering Therapy
WARNINGS,Myopathy/RhabdomyolysisandPRECAUTIONS,Drug Interactions).
Dosage in Patients with Renal Insufficiency
CLINICAL PHARMACOLOGYandWARNINGS,Myopathy/Rhabdomyolysis).
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION


LovastatinLovastatin TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!