LubriFresh P.M.
Major Pharaceuticals
Bausch & Lomb Incorporated
Artificial Tears Ointment Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- LubriFresh P.M. Uses
- Warnings
- Directions
- LubriFresh P.M. Other information
- Inactive ingredient
- Questions or comments?
- Package/Label Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredient
Mineral oil 15%
White petrolatum 83%
Purpose
Lubricant
LubriFresh P.M. Uses
- to prevent further irritation
- to relieve dryness of the eye
Warnings
When using this product
- replace cap after use
- do not touch tip of container to any surface to avoid contamination
Stop use and ask a doctor if
- condition worsens or persists for more than 72 hours
- you experience eye pain, changes in vision, continued redness or irritation of the eye
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
-
-
-
LubriFresh P.M. Other information
- store at 15° - 30°C (59° - 86°F)
- keep tightly closed
- see crimp of tube or carton for Lot Number and Expiration Date
Inactive ingredient
lanolin oil
Questions or comments?
Serious side effects associated with use of this product may be reported to 1800-323-0000
*Major LubriFreshTM P.M. is not manufactured or distributed by Allergan Inc., distributor of Refresh P.M.®
Package/Label Principal Display Panel
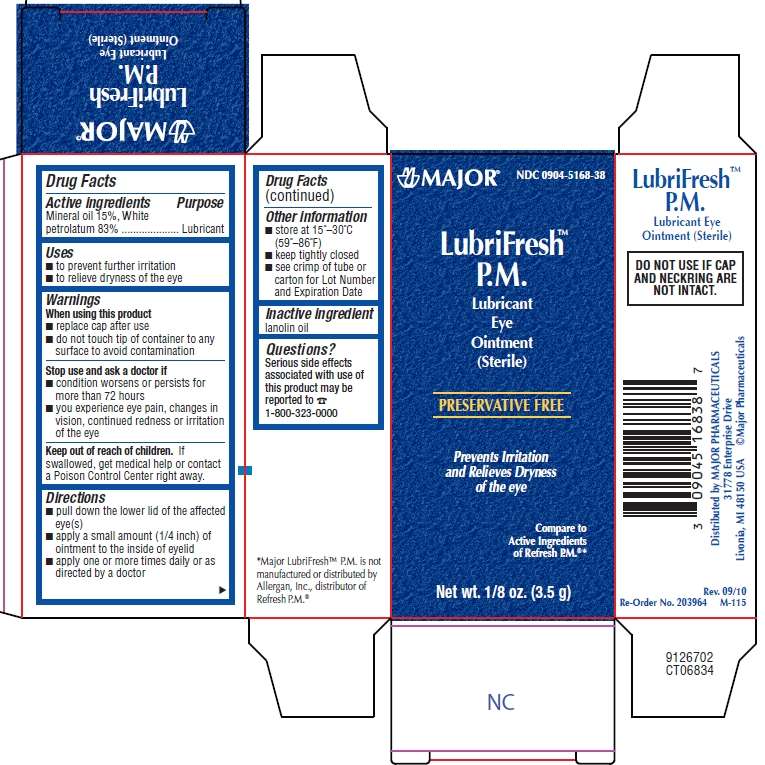
NDC 0904-5168-38
Major®
LubriFreshTM P.M.
Lubricant Eye Ointment (Sterile)
PRESERVATIVE FREE
Prevents Irritation and Relieves Dryness of the eye
Compare to Active Ingredients of Refresh P.M.®*
Net Wt. 1/8 oz (3.5 g)
LubriFresh P.M.white petrolatum mineral oil OINTMENT
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!