Lupron Depot
LUPRON DEPOT 7.5 mg(leuprolide acetate for depot suspension)
FULL PRESCRIBING INFORMATION: CONTENTS*
- LUPRON DEPOT DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL STUDIES
- indications and usage
- LUPRON DEPOT CONTRAINDICATIONS
- warnings
- PRECAUTIONS
- Side Effects
- overdosage
- LUPRON DEPOT DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
FULL PRESCRIBING INFORMATION
Rx only
LUPRON DEPOT DESCRIPTION
Leuprolide acetate is a synthetic nonapeptide analog of naturally occurring gonadotropin-releasing hormone (GnRH or LH-RH). The analog possesses greater potency than the natural hormone. The chemical name is 5-oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L-tyrosyl-D-leucyl-L-leucyl-L-arginyl-N-ethyl-L-prolinamide acetate (salt) with the following structural formula:
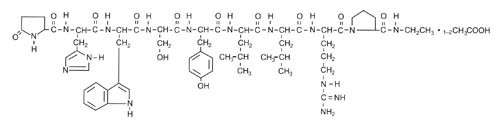
LUPRON DEPOT is available in a prefilled dual-chamber syringe containing sterile lyophilized microspheres which, when mixed with diluent, becomes a suspension intended as a monthly intramuscular injection.
The front chamber of LUPRON DEPOT 7.5 mg for 1-month administration prefilled dual-chamber syringe contains leuprolide acetate (7.5 mg), purified gelatin (1.3 mg), DL-lactic and glycolic acids copolymer (66.2 mg), and D-mannitol (13.2 mg). The second chamber of diluent contains carboxymethylcellulose sodium (5 mg), D-mannitol (50 mg), polysorbate 80 (1 mg), water for injection, USP, and glacial acetic acid, USP to control pH.
During the manufacture of LUPRON DEPOT 7.5 mg for 1-month administration, acetic acid is lost, leaving the peptide.
CLINICAL PHARMACOLOGY
Mechanism of Action
Leuprolide acetate, a GnRH agonist, acts as a potent inhibitor of gonadotropin secretion. Animal studies indicate that following an initial stimulation, chronic administration of leuprolide acetate results in suppression of ovarian and testicular steroidogenesis. This effect is reversible upon discontinuation of drug therapy.
Administration of leuprolide acetate has resulted in inhibition of the growth of certain hormone dependent tumors (prostatic tumors in Noble and Dunning male rats and DMBA-induced mammary tumors in female rats) as well as atrophy of the reproductive organs.
Pharmacodynamics
In humans, administration of leuprolide acetate results in an initial increase in circulating levels of luteinizing hormone (LH) and follicle stimulating hormone (FSH), leading to a transient increase in levels of the gonadal steroids (testosterone and dihydrotestosterone in males, and estrone and estradiol in premenopausal females). However, continuous administration of leuprolide acetate results in decreased levels of LH and FSH. In males, testosterone is reduced to castrate levels. In premenopausal females, estrogens are reduced to postmenopausal levels. These decreases occur within two to four weeks after initiation of treatment, and castrate levels of testosterone in prostatic cancer patients have been demonstrated for more than five years.
Leuprolide acetate is not active when given orally.
Pharmacokinetics
Absorption
Following a single injection of LUPRON DEPOT 7.5 mg for 1-month administration to patients, mean plasma leuprolide concentration was almost 20 ng/mL at 4 hours and 0.36 ng/mL at 4 weeks. However, intact leuprolide and an inactive major metabolite could not be distinguished by the assay which was employed in the study. Nondetectable leuprolide plasma concentrations have been observed during chronic LUPRON DEPOT 7.5 mg administration, but testosterone levels appear to be maintained at castrate levels.
Distribution
The mean steady-state volume of distribution of leuprolide following intravenous bolus administration to healthy male volunteers was 27 L. In vitro binding to human plasma proteins ranged from 43% to 49%.
Metabolism
In healthy male volunteers, a 1 mg bolus of leuprolide administered intravenously revealed that the mean systemic clearance was 7.6 L/h, with a terminal elimination half-life of approximately 3 hours based on a two compartment model.
In rats and dogs, administration of 14C-labeled leuprolide was shown to be metabolized to smaller inactive peptides, a pentapeptide (Metabolite I), tripeptides (Metabolites II and III) and a dipeptide (Metabolite IV). These fragments may be further catabolized.
The major metabolite (M-I) plasma concentrations measured in 5 prostate cancer patients reached maximum concentration 2 to 6 hours after dosing and were approximately 6% of the peak parent drug concentration. One week after dosing, mean plasma M-I concentrations were approximately 20% of mean leuprolide concentrations.
Excretion
Following administration of LUPRON DEPOT 3.75 mg to 3 patients, less than 5% of the dose was recovered as parent and M-I metabolite in the urine.
Special Populations
The pharmacokinetics of the drug in hepatically and renally impaired patients have not been determined.
CLINICAL STUDIES
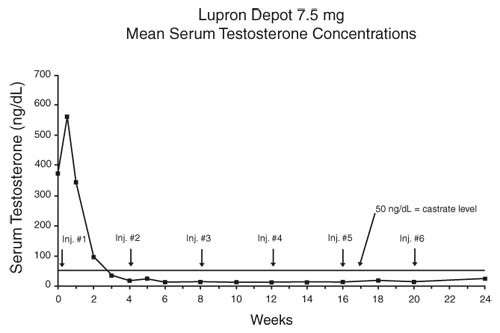
In an open-label, non-comparative, multicenter clinical study of LUPRON DEPOT 7.5 mg for 1-month administration, 56 patients with stage D2 prostatic adenocarcinoma and no prior systemic treatment were enrolled. The objectives were to determine if a 7.5 mg depot formulation of leuprolide injected once every 4 weeks would reduce and maintain serum testosterone to castrate range (≤50 ng/dL), to evaluate objective clinical response, and to assess the safety of the formulation. During the initial 24 weeks, serum testosterone was measured weekly, biweekly, or every four weeks and objective tumor response assessments were performed at Weeks 12 and 24. Once the patient completed the initial 24-week treatment phase, treatment continued at the investigator's discretion. Data from the initial 24-week treatment phase are summarized in this section.
In the majority of patients, serum testosterone increased by 50% or more above baseline during the first week of treatment. Serum testosterone suppressed to the castrate range within 30 days of the initial depot injection in 94% (51/54) of patients for whom testosterone suppression was achieved (2 patients withdrew prior to onset of suppression) and within 66 days in all 54 patients. Mean serum testosterone suppressed to castrate level by Week 3. The median dosing interval between injections was 28 days. One escape from suppression (2 consecutive testosterone values greater than 50 ng/dL after achieving castrate level) was noted at Week 18, associated with a substantial dosing delay. In this patient, serum testosterone returned to the castrate range at the next monthly measurement. Serum testosterone was minimally above the castrate range on a single occasion for 4 other patients. No clinical significance was attributed to these rises in testosterone.
Secondary efficacy endpoints evaluated included objective tumor response, assessed by clinical evaluations of tumor burden (complete response, partial response, objectively stable, and progression), as well as changes in local disease status, assessed by digital rectal examination, and changes in prostatic acid phosphatase (PAP). These evaluations were performed at Weeks 12 and 24. The objective tumor response analysis showed a "no progression" (ie. complete or partial response, or stable disease) in 77% (40/52) of patients at Week 12, and in 84% (42/50) of patients at Week 24. Local disease improved or remained stable in all (42) patients evaluated at Week 12 and in 98% (41/42) of patients elevated at Week 24. PAP normalized or decreased at Week 12 and/or 24 in the majority of patients with elevated baseline PAP.
Periodic monitoring of serum testosterone and PSA levels is recommended, especially if the anticipated clinical or biochemical response to treatment has not been achieved. It should be noted that results of testosterone determinations are dependent on assay methodology. It is advisable to be aware of the type and precision of the assay methodology to make appropriate clinical and therapeutic decisions.
indications and usage
LUPRON DEPOT 7.5 mg for 1-month administration is indicated in the palliative treatment of advanced prostatic cancer.
LUPRON DEPOT CONTRAINDICATIONS
- LUPRON DEPOT is contraindicated in individuals with known hypersensitivity to GnRH agonists or any of the excipients in LUPRON DEPOT. Reports of anaphylactic reactions to GnRH agonist analogs have been reported in the medical literature.
- LUPRON DEPOT may cause fetal harm when administered to a pregnant woman. Expected hormonal changes that occur with LUPRON DEPOT increase the risk for pregnancy loss and fetal harm when administered to a pregnant woman (see Pregnancy Category X). LUPRON DEPOT is contraindicated in women who are or may become pregnant. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
warnings
Tumor Flare
Initially, LUPRON DEPOT, like other GnRH agonists, causes increases in serum levels of testosterone to approximately 50% above baseline during the first week of treatment. Isolated cases of ureteral obstruction and spinal cord compression have been observed, which may contribute to paralysis with or without fatal complications. Transient worsening of symptoms may develop. A small number of patients may experience a temporary increase in bone pain, which can be managed symptomatically.
PRECAUTIONS
Information for Patients
- If they experience an allergic reaction to other drugs like LUPRON DEPOT, they should not use this drug.
- The most common side effects associated with LUPRON DEPOT are hot flashes, pain (especially joint pain and back pain), injection site pain and fatigue.
- LUPRON DEPOT may cause impotence.
- The increase in testosterone that occurs during the first weeks of therapy can cause an increase in urinary symptoms or pain.
- If they have metastatic cancer to the spine or urinary tract, they need close medical attention during the first weeks of therapy.
- They should notify their doctor if they develop new or worsened symptoms after beginning LUPRON DEPOT treatment.
General
Patients with metastatic vertebral lesions and/or with urinary tract obstruction should be closely observed during the first few weeks of therapy (see WARNINGS section).
Hyperglycemia and Diabetes
Hyperglycemia and an increased risk of developing diabetes have been reported in men receiving GnRH agonists. Hyperglycemia may represent development of diabetes mellitus or worsening of glycemic control in patients with diabetes. Monitor blood glucose and/or glycosylated hemoglobin (HbA1c) periodically in patients receiving a GnRH agonist and manage with current practice for treatment of hyperglycemia or diabetes.
Cardiovascular Diseases
Increased risk of developing myocardial infarction, sudden cardiac death and stroke has been reported in association with use of GnRH agonists in men. The risk appears low based on the reported odds ratios, and should be evaluated carefully along with cardiovascular risk factors when determining a treatment for patients with prostate cancer. Patients receiving a GnRH agonist should be monitored for symptoms and signs suggestive of development of cardiovascular disease and be managed according to current clinical practice.
Effect on QT/QTc Interval
Long-term androgen deprivation therapy prolongs the QT interval. Physicians should consider whether the benefits of androgen deprivation therapy outweigh the potential risks in patients with congenital long QT syndrome, electrolyte abnormalities, or congestive heart failure and in patients taking class IA (e.g., quinidine, procainamide) or Class III (e.g., amiodarone, sotalol) antiarrhythmic medications.
Postmarketing reports of convulsions have been observed in patients on leuprolide acetate therapy. These included patients with a history of seizures, epilepsy, cerebrovascular disorders, central nervous system anomalies or tumors, and in patients on concomitant medications that have been associated with convulsions such as bupropion and SSRIs. Convulsions have also been reported in patients in the absence of any of the conditions mentioned above. Patients receiving a GnRH agonist who experience convulsions should be managed according to current clinical practice.
Laboratory Tests
Response to LUPRON DEPOT 7.5 mg for 1-month administration should be monitored by measuring serum levels of testosterone. In the majority of patients, testosterone levels increased above baseline, declining thereafter to castration levels (<50 ng/dL) within four weeks (see CLINICAL STUDIES section).
Drug Interactions
No pharmacokinetic-based drug-drug interaction studies have been conducted with LUPRON DEPOT. However, because leuprolide acetate is a peptide that is primarily degraded by peptidase and not by Cytochrome P-450 enzymes as noted in specific studies, and the drug is only about 46% bound to plasma proteins, drug interactions would not be expected to occur (see Pharmacokinetics ).
Drug/Laboratory Test Interactions
Administration of LUPRON DEPOT in therapeutic doses results in suppression of the pituitary-gonadal system. Normal function is usually restored within three months after treatment is discontinued. Due to the suppression of the pituitary-gonadal system by LUPRON DEPOT, diagnostic tests of pituitary gonadotropic and gonadal functions conducted during treatment and for up to three months after discontinuation of LUPRON DEPOT may be affected.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year carcinogenicity studies were conducted in rats and mice. In rats, a dose-related increase of benign pituitary hyperplasia and benign pituitary adenomas was noted at 24 months when the drug was administered subcutaneously at high daily doses (0.6 to 4 mg/kg). There was a significant but not dose-related increase of pancreatic islet-cell adenomas in females and of testicular interstitial cell adenomas in males (highest incidence in the low dose group). In mice, no leuprolide acetate-induced tumors or pituitary abnormalities were observed at a dose as high as 60 mg/kg for two years. Patients have been treated with leuprolide acetate for up to three years with doses as high as 10 mg/day and for two years with doses as high as 20 mg/day without demonstrable pituitary abnormalities.
Genotoxicity studies were conducted with leuprolide acetate using bacterial and mammalian systems. These studies provided no evidence of a mutagenic potential or chromosomal aberrations.
Leuprolide may reduce male and female fertility. Administration of leuprolide acetate to male and female rats at dose of 0.024, 0.24, and 2.4 mg/kg as monthly depot formulation for up to 3 months (approximately as low as 1/30 of the human dose based on body surface area using an estimated daily dose in animals and humans) caused atrophy of the reproductive organs, and suppression of reproductive function. These changes were reversible upon cessation of treatment. Clinical and pharmacologic studies in adults (≥ 18 years) with leuprolide acetate and similar analogs have shown reversibility of fertility suppression when the drug is discontinued after continuous administration for periods of up to 24 weeks.
Clinical and pharmacologic studies in adults (≥ 18 years) with leuprolide acetate and similar analogs have shown reversibility of fertility suppression when the drug is discontinued after continuous administration for periods of up to 24 weeks.
Pregnancy Category X
See CONTRAINDICATIONS section.
LUPRON DEPOT is contraindicated in women who are or may become pregnant while receiving the drug. Expected hormonal changes that occur with LUPRON DEPOT treatment increase the risk for pregnancy loss and fetal harm when administered to a pregnant woman. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
Major fetal abnormalities were observed in rabbits after a single administration of the monthly formulation of LUPRON DEPOT on day 6 of pregnancy at doses of 0.00024, 0.0024, and 0.024 mg/kg (approximately 1/1600 to 1/16 the human dose based on body surface area using an estimated daily dose in animals and humans). Since a depot formulation was utilized in the study, a sustained exposure to leuprolide was expected throughout the period of organogenesis and to the end of gestation. Similar studies in rats did not demonstrate an increase in fetal malformations, however, there was increased fetal mortality and decreased fetal weights with the two higher doses of the monthly formulation of LUPRON DEPOT in rabbits and with the highest dose (0.024 mg/kg) in rats.
Nursing Mothers
LUPRON DEPOT is not indicated for women (see INDICATIONS AND USAGE section). It is not known whether leuprolide is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from LUPRON DEPOT, a decision should be made to discontinue nursing or discontinue the drug taking into account the importance of the drug to the mother.
Pediatric Use
See LUPRON DEPOT-PED® (leuprolide acetate for depot suspension) labeling for the safety and effectiveness in children with central precocious puberty.
Geriatric Use
In the clinical trials for LUPRON DEPOT in prostate cancer, the majority (80%) of the subjects studied were at least 65 years of age. Therefore, the labeling reflects the pharmacokinetics, efficacy and safety of LUPRON DEPOT in this population.
Side Effects
Clinical Trials
In the majority of patients testosterone levels increased above baseline during the first week, declining thereafter to baseline levels or below by the end of the second week of treatment.
Potential exacerbations of signs and symptoms during the first few weeks of treatment is a concern in patients with vertebral metastases and/or urinary obstruction or hematuria which, if aggravated, may lead to neurological problems such as temporary weakness and/or paresthesia of the lower limbs or worsening of urinary symptoms (see WARNINGS section).
In a clinical trial of LUPRON DEPOT 7.5 mg for 1-month administration, the following adverse reactions were reported in 5% or more of the patients during the initial 24-week treatment period regardless of causality.
| LUPRON DEPOT 7.5 mg for 1-Month Administration (N=56) | ||
| N | (%) | |
| Body as a Whole | ||
| General pain | 13 | (23.2) |
| Infection | 3 | (5.4) |
| Cardiovascular System | ||
| Hot flashes/sweats* | 32 | (57.1) |
| Digestive System | ||
| GI disorders | 8 | (14.3) |
| Metabolic and Nutritional Disorders | ||
| Edema | 8 | (14.3) |
| Nervous System | ||
| Libido decreased* | 3 | (5.4) |
| Respiratory System | ||
| Respiratory disorder | 6 | (10.7) |
| Urogenital System | ||
| Urinary disorder | 7 | (12.5) |
| Impotence* | 3 | (5.4) |
| Testicular atrophy* | 3 | (5.4) |
| * Due to the expected physiologic effect of decreased testosterone levels. | ||
In this same study, the following adverse reactions were reported in less than 5% of the patients on LUPRON DEPOT 7.5 mg for 1-month administration.
Body as a Whole - Asthenia, Cellulitis, Fever, Headache, Injection site reaction, Neoplasm; Cardiovascular System - Angina, Congestive heart failure; Digestive System - Anorexia, Dysphagia, Eructation, Peptic ulcer; Hemic and Lymphatic System - Ecchymosis; Musculoskeletal System - Myalgia; Nervous System - Agitation, Insomnia/sleep disorders, Neuromuscular disorders; Respiratory System - Emphysema, Hemoptysis, Lung edema, Sputum increased; Skin and Appendages - Hair disorder, Skin reaction; Urogenital System - Balanitis, Breast enlargement, Urinary tract infection.
Laboratory: Abnormalities of certain parameters were observed, but their relationship to drug treatment are difficult to assess in this population. The following were recorded in ≥5% of patients at final visit: Decreased albumin, decreased hemoglobin/hematocrit, decreased prostatic acid phosphatase, decreased total protein, decreased urine specific gravity, hyperglycemia, hyperuricemia, increased BUN, increased creatinine, increased liver function tests (AST, LDH), increased phosphorus, increased platelets, increased prostatic acid phosphatase, increased total cholesterol, increased urine specific gravity, leukopenia.
Postmarketing
The following adverse reactions have been identified during postapproval use of LUPRON DEPOT. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
During postmarketing surveillance, which includes other dosage forms and other patient populations, the following adverse events were reported.
Like other drugs in this class, mood swings, including depression, have been reported. There have been very rare reports of suicidal ideation and attempt. Many, but not all, of these patients had a history of depression or other psychiatric illness. Patients should be counseled on the possibility of development or worsening of depression during treatment with LUPRON.
Symptoms consistent with an anaphylactoid or asthmatic process have been rarely (incidence rate of about 0.002%) reported. Rash, urticaria, and photosensitivity reactions have also been reported.
Changes in Bone Density: Decreased bone density has been reported in the medical literature in men who have had orchiectomy or who have been treated with an LH-RH agonist analog. In a clinical trial, 25 men with prostate cancer, 12 of whom had been treated previously with leuprolide acetate for at least six months, underwent bone density studies as a result of pain. The leuprolide-treated group had lower bone density scores than the nontreated control group. It can be anticipated that long periods of medical castration in men will have effects on bone density.
Pituitary apoplexy: During post-marketing surveillance, rare cases of pituitary apoplexy (a clinical syndrome secondary to infarction of the pituitary gland) have been reported after the administration of gonadotropin-releasing hormone agonists. In a majority of these cases, a pituitary adenoma was diagnosed, with a majority of pituitary apoplexy cases occurring within 2 weeks of the first dose, and some within the first hour. In these cases, pituitary apoplexy has presented as sudden headache, vomiting, visual changes, ophthalmoplegia, altered mental status, and sometimes cardiovascular collapse. Immediate medical attention has been required.
Localized reactions including induration and abscess have been reported at the site of injection.
Symptoms consistent with fibromyalgia (eg, joint and muscle pain, headaches, sleep disorders, gastrointestinal distress, and shortness of breath) have been reported individually and collectively.
Cardiovascular System – Hypotension, Myocardial infarction, Pulmonary embolism;
Respiratory, thoracic and mediastinal disorder – Interstitial lung disease;
Hepato-biliary disorder: Serious drug-induced liver injury
Hemic and Lymphatic System – Decreased WBC;
Central/Peripheral Nervous System – Convulsion, Peripheral neuropathy, Spinal fracture/paralysis;
Endocrine System – Diabetes;
Musculoskeletal System – Tenosynovitis-like symptoms;
Urogenital System – Prostate pain.
See other LUPRON DEPOT and LUPRON Injection package inserts for other events reported in women and pediatric populations.
overdosage
There is no experience of overdosage in clinical trials. In rats, a single subcutaneous dose of 100 mg/kg (approximately 4,000 times the estimated daily human dose based on body surface area), resulted in dyspnea, decreased activity, and excessive scratching. In early clinical trials with daily subcutaneous leuprolide acetate, doses as high as 20 mg/day for up to two years caused no adverse effects differing from those observed with the 1 mg/day dose.
LUPRON DEPOT DOSAGE AND ADMINISTRATION
LUPRON DEPOT must be administered under the supervision of a physician.
The recommended dose of LUPRON DEPOT is 7.5 mg for 1-month administration, incorporated in a depot formulation. Due to different release characteristics, a fractional dose, or a combination of doses of this depot formulation is not equivalent to the same dose of the monthly formulation and should not be given.
Incorporated in a depot formulation, the lyophilized microspheres are to be reconstituted and administered every 4 weeks as a single intramuscular injection.
For optimal performance of the prefilled dual chamber syringe (PDS), read and follow the following instructions:
- The lyophilized microspheres are to be reconstituted and administered as a single intramuscular injection.
- Since LUPRON DEPOT does not contain a preservative, the suspension should be injected immediately or discarded if not used within two hours.
- As with other drugs administered by injection, the injection site should be varied periodically.
- The LUPRON DEPOT powder should be visually inspected and the syringe should NOT BE USED if clumping or caking is evident. A thin layer of powder on the wall of the syringe is considered normal prior to mixing with the diluent. The diluent should appear clear.
- To prepare for injection, screw the white plunger into the end stopper until the stopper begins to turn.
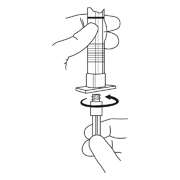
- Hold the syringe UPRIGHT. Release the diluent by SLOWLY PUSHING (6 to 8 seconds) the plunger until the first stopper is at the blue line in the middle of the barrel.
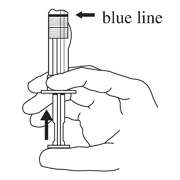
- Keep the syringe UPRIGHT. Gently mix the microspheres (powder) thoroughly to form a uniform suspension. The suspension will appear milky. If the powder adheres to the stopper or caking/clumping is present, tap the syringe with your finger to disperse. DO NOT USE if any of the powder has not gone into suspension.
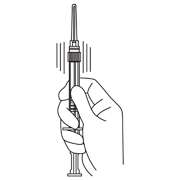
- Hold the syringe UPRIGHT. With the opposite hand pull the needle cap upward without twisting.
- Keep the syringe UPRIGHT. Advance the plunger to expel the air from the syringe.
- After cleaning the injection site with an alcohol swab, insert the needle completely at a 90 degree angle.
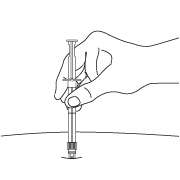
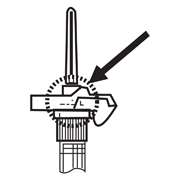
NOTE: Aspirated blood would be visible just below the luer lock connection if a blood vessel is accidentally penetrated. If present, blood can be seen through the transparent LuproLoc® safety device. If blood is present remove the needle immediately. Do not inject the medication.
- Inject the entire contents of the syringe intramuscularly at the time of reconstitution. The suspension settles very quickly following reconstitution; therefore, LUPRON DEPOT should be mixed and used immediately.
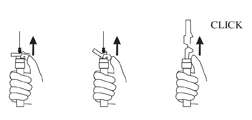
AFTER INJECTION - Withdraw the needle. Immediately activate the LuproLoc® safety device by pushing the arrow forward with the thumb or finger, as illustrated, until the device is fully extended and a CLICK is heard or felt.
HOW SUPPLIED
Each LUPRON DEPOT 7.5 mg for 1-month administration kit (NDC 0074-3642-03) contains:
- one prefilled dual-chamber syringe containing needle with LuproLoc® safety device
- one plunger
- two alcohol swabs
- a complete prescribing information enclosure
The prefilled dual-chamber syringe contains sterile lyophilized microspheres of leuprolide acetate incorporated in a biodegradable lactic acid/glycolic acid copolymer. When mixed with 1 mL of accompanying diluent, LUPRON DEPOT 7.5 mg for 1-month administration is administered as a single monthly intramuscular injection.
Store at 25°C (77°F); excursions permitted to 15–30°C (59–86°F) [See USP Controlled Room Temperature]
REFERENCES
- NIOSH Alert: Preventing occupational exposures to antineoplastic and other hazardous drugs in healthcare settings. 2004. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2004-165.
- OSHA Technical Manual, TED 1-0.15A, Section VI: Chapter 2. Controlling Occupational Exposure to Hazardous Drugs. OSHA, 1999. http://www.osha.gov/dts/osta/otm/otm_vi/otm_vi_2.html
- American Society of Health-System Pharmacists. ASHP guidelines on handling hazardous drugs. Am J Health-Syst Pharm. 2006; 63; 1172-1193.
- Polovich, M., White, J.M., & Kelleher, L.O. (eds.) 2005. Chemotherapy and biotherapy guidelines and recommendations for practice (2nd Ed.) Pittsburgh, PA: Oncology Nursing Society.
Manufactured for
AbbVie Inc.
North Chicago, IL 60064
By Takeda Pharmaceutical Company Limited
Osaka, JAPAN 540-8645
03-A852 July, 2013
NDC 0074–3642–03
FOR ADULT USE 7.5 mg for 1–month administration
Single Dose Administration Kit with prefilled dual-chamber syringe.
LupronDepot®
(Leuprolide Acetate for Depot Suspension)
7.5 mg for 1–month administration
FOR INTRAMUSCULAR INJECTION
The front chamber contains: leuprolide acetate 7.5 mg • purified gelatin 1.3 mg
• DL-lactic and glycolic acids copolymer 66.2 mg • D-mannitol 13.2 mg
The second chamber contains: carboxymethylcellulose sodium 5 mg
• D-mannitol 50 mg • polysorbate 80 1 mg • water for injection,
USP, and glacial acetic acid, USP to control pH
Rx only

Lupron Depotleuprolide acetate KIT
| ||||||||||||||||||||||||||||||||||||||||