Mannitol
Mannitol I.V.Mannitol Injection, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- MANNITOL DESCRIPTION
- CLINICAL PHARMACOLOGY
- MANNITOL INDICATIONS AND USAGE
- MANNITOL CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- MANNITOL ADVERSE REACTIONS
- OVERDOSAGE
- MANNITOL DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Flexible Plastic Container
Fliptop Vial
Rx only
MANNITOL DESCRIPTION
Mannitol I.V. (Mannitol Injection, USP) is a sterile, nonpyrogenic solution of mannitol in water for injection available in concentrations of 5%, 10%, 15%, 20% in flexible plastic containers and 25% in a fliptop vial for administration by intravenous infusion only.
The content and characteristics of the available concentrations are as follows:
|
Conc. (%) |
g/100 mL |
mOsmol/liter (calc.) |
pH* |
|
5 |
5 |
274 |
6.3 (4.5 to 7.0) |
|
10 |
10 |
549 |
6.3 (4.5 to 7.0) |
|
15 |
15 |
823 |
6.3 (4.5 to 7.0) |
|
20 |
20 |
1098 |
6.3 (4.5 to 7.0) |
|
25 |
25 |
1372 |
5.9 (4.5 to 7.0) |
*Concentrations up to 20% may contain sodium bicarbonate for pH adjustment; the 25% concentration may contain sodium bicarbonate and/or hydrochloric acid for pH adjustment.
The solutions contain no bacteriostat, antimicrobial agent or added buffer (except for pH adjustment) and each is intended only as a single-dose injection. When smaller doses are required the unused portion should be discarded.
Mannitol Injection, USP is a parenteral obligatory osmotic diuretic.
Mannitol, USP is chemically designated D-mannitol (C6H14O6), a white crystalline powder or free-flowing granules freely soluble in water. It has the following structural formula:
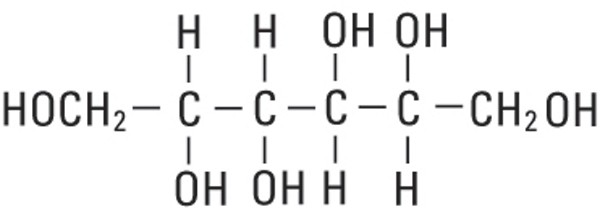
Water for Injection, USP is chemically designated H20.
The flexible plastic container is fabricated from a specially formulated polyvinylchloride. Water can permeate from inside the container into the overwrap, but not in amounts sufficient to affect the solution significantly. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials. Exposure to temperatures above 25°C/77°F during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period.
CLINICAL PHARMACOLOGY
When administered intravenously mannitol is confined to the extracellular space, only slightly metabolized and rapidly excreted by the kidney. Approximately 80% of a 100 g dose appears in the urine in 3 hours. The drug is freely filtered by the glomeruli with less than 10% tubular reabsorption; it is not secreted by tubular cells. Mannitol induces diuresis by elevating the osmolarity of the glomerular filtrate and thereby hindering tubular reabsorption of water. Excretion of sodium and chloride is also enhanced.
MANNITOL INDICATIONS AND USAGE
Mannitol I.V. (Mannitol Injection, USP) is indicated for the following purposes in adults and pediatric patients.
Therapeutic Use
-
-
-
-
Diagnostic Use
Measurement of glomerular filtration rate.
MANNITOL CONTRAINDICATIONS
-
-
-
-
-
-
-
WARNINGS
-
-
-
-
-
-
-
-
PRECAUTIONS
-
-
-
-
-
-
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Studies with solutions from flexible plastic containers have not been performed to evaluate carcinogenic potential, mutagenic potential or effects on fertility.
Pregnancy Category C.
Animal reproduction studies have not been conducted with mannitol injection. It is also not known whether mannitol injection can cause fetal harm when given to a pregnant woman or can affect reproduction. Mannitol injection should be given to a pregnant woman only if clearly needed.
Nursing Mothers:
Caution should be exercised when solutions from flexible plastic containers are administered to a nursing mother.
Pediatric Use:
(See DOSAGE AND ADMINISTRATION sections.) Safety and effectiveness of solutions from flexible plastic containers in pediatric patients have not been well established.
MANNITOL ADVERSE REACTIONS
Adverse reactions more commonly reported during or after the infusion of mannitol include: Pulmonary congestion, fluid and electrolyte imbalance, acidosis, electrolyte loss, dryness of mouth, thirst, marked diuresis, urinary retention, edema, headache, blurred vision, convulsions, nausea, vomiting, rhinitis, arm pain, skin necrosis, thrombophlebitis, chills, dizziness, urticaria, dehydration, hypotension, tachycardia, fever and angina-like chest pains.
Reactions which may occur because of the solution or the technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation and hypervolemia.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
OVERDOSAGE
Too rapid infusion of large amounts of mannitol will cause a shift of intracellular water into the extracellular compartment resulting in cellular dehydration and overexpansion of the intravascular space with hyponatremia, congestive heart failure and pulmonary edema. Repeated doses should not be given to patients with persistent oliguria as this can produce a hyperosmolar state and precipitate congestive heart failure and pulmonary edema due to volume overload. Dosage must be carefully monitored and adjusted in accordance with the clinical situation to avoid the consequences of overdosage. (See CONTRAINDICATIONS , WARNINGS , PRECAUTIONS and DOSAGE AND ADMINISTRATION ).
MANNITOL DOSAGE AND ADMINISTRATION
Mannitol I.V. (Mannitol Injection, USP) should be administered only by intravenous infusion. The total dosage, concentration and rate of administration should be governed by the nature and severity of the condition being treated, fluid requirement and urinary output. The usual adult dosage ranges from 50 to 200 g in a 24-hour period, but in most instances an adequate response will be achieved at a dosage of approximately 100 g/24 hours. The rate of administration is usually adjusted to maintain a urine flow of at least 30 to 50 mL/hr. The total dose should be adjusted according to the clinical response and adverse events (see WARNINGS ).
Test Dose: A test dose of mannitol should be given prior to instituting Mannitol I.V. therapy for patients with marked oliguria or those believed to have inadequate renal function. In adults the dose is 0.2 g/kg body weight. In pediatric patients the dose is 0.2 g/kg body weight or 6 g/m2 body surface area. The infusion is given as a 15% to 25% solution over a period of 3 to 5 minutes to produce a urine flow of at least 30 to 50 mL/hour. If urine flow does not increase, a second dose may be given; but if there is inadequate response, the patient should be re-evaluated.
Prevention of Acute Renal Failure (Oliguria): When used during cardiovascular or other types of surgery, 50 to 100 g of mannitol as a 5%, 10%, or 15% solution may be given. The concentration will depend on the fluid requirements of the patient.
Treatment of Oliguria: The usual dose to promote diuresis in oliguric patients: Adults, 300 to 400 mg/kg of body weight (21 to 28 g for a 70 kg patient) or up to 100 g of solution, given as a single dose (often in conjunction with furosemide); pediatric patients, 0.25 to 2 g/kg body weight or 60 g/m2 body surface area as a 15% to 20% solution over a period of 2 to 6 hours. Doses should not be repeated in patients with persistent oliguria.
Reduction of Intracranial Pressure and Brain Mass: In adults a dose of 0.25 to 2 g/kg body weight as a 15% to 25% solution administered over a period of 30 to 60 minutes; pediatric patients 1 to 2 g/kg body weight or 30 to 60 g/m2 body surface area over a period of 30 to 60 minutes. In small or debilitated patients, a dose of 500 mg/kg may be sufficient. Careful evaluation must be made of the circulatory and renal reserve prior to and during administration of mannitol at the higher doses and rapid infusion rates. Careful attention must be paid to fluid and electrolyte balance, body weight, and total input and output before and after infusion of mannitol. Evidence of reduced cerebral spinal fluid pressure must be observed within 15 minutes after starting infusion.
Reduction of Intraocular Pressure: In adults a dose of 0.25 to 2 g/kg body weight as a 15% to 25% solution administered over a period of 30 to 60 minutes; pediatric patients 1 to 2 g/kg body weight or 30 to 60 g/m2 body surface area over a period of 30 to 60 minutes. In small or debilitated patients, a dose of 500 mg/kg may be sufficient. When used preoperatively, the dose should be given one to one and one-half hours before surgery to achieve maximal reduction of intraocular pressure before operation.
Adjunctive Therapy for Intoxications: As an agent to promote urinary excretion of toxic substances: Adults may receive a 5% to 25% solution for as long as indicated if urinary output remains high; pediatric patients may receive 2 g/kg of body weight of a 5% or 10% solution. The concentration will depend upon the fluid requirement and urinary output of the patient. If benefits are not observed after 200 g of mannitol are administered, discontinue the mannitol therapy. Intravenous water and electrolytes must be given to match the loss of these substances in the urine, sweat and expired air.
Measurement of Glomerular Filtration Rate (GFR): 100 mL of a 20% solution (20 g) should be diluted with 180 mL of sodium chloride injection (normal saline) or 200 mL of a 10% solution (20 g) should be diluted with 80 mL of sodium chloride injection (normal saline). The resulting 280 mL of 7.2% solution is infused at a rate of 20 mL per minute. The urine is collected by catheter for a specific period of time and analyzed for mannitol excreted in mg per minute. A blood sample is drawn at the start and at the end of the time period and the concentration of mannitol determined in mg/mL of plasma. GFR is the number of mL of plasma that must have been filtered to account for the amount excreted per minute in the urine. Normal clearance rates are approximately 125 mL/minute for men; 116 mL/minute for women.
Drug Interactions
Additives may be incompatible. Consult with pharmacist, if available. When introducing additives to the flexible container, use aseptic technique, mix thoroughly and do not store.
Do not place 25% Mannitol Injection, USP in polyvinylchloride bags; a white flocculent precipitate may form from contact with PVC surfaces. Parenteral drug products should be inspected visually for particulate matter and discoloration; whenever container and solution permit. (See PRECAUTIONS ).
INSTRUCTIONS FOR USE - Flexible Container
To Open
Tear outer wrap at notch and remove solution container. If supplemental medication is desired, follow directions below before preparing for administration. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
To Add Medication
-
-
-
-
Preparation for Administration
(Use aseptic technique)
-
-
-
-
-
-
-
-
WARNING: Do not use flexible container in series connections.
INSTRUCTlONS FOR USE - Fliptop Vial
Remove cover and cleanse stopper with antiseptic before use.
HOW SUPPLIED
Mannitol I.V. (Mannitol Injection, USP) is supplied in single-dose containers as follows:
|
NDC No. |
Conc. % |
Size (mL) |
|
0409–7712–09 |
5 |
1000 Flexible Container |
|
0409–7713–09 |
10 |
1000 Flexible Container |
|
0409–7714–03 |
15 |
500 Flexible Container |
|
0409–7715–02 |
20 |
250 Flexible Container |
|
0409–7715–03 |
20 |
500 Flexible Container |
|
0409–4031–01 |
25 |
50 Fliptop Vial |
NOTE : Crystals may form in mannitol solutions especially if the solutions are chilled. To dissolve crystals in the flexible container, warm the unit to 70°C with agitation. Heat solution by using a dry-heat cabinet with overwrap intact. The use of a water bath is not recommended. To dissolve the crystals in the fliptop vial, warm the bottle in hot water at 80°C and periodically shake vigorously. 25% Mannitol Injection, USP may be autoclaved at 121°C for 20 minutes at 15 psi. Remove cover from fliptop vial and cleanse stopper with antiseptic before use. Cool to body temperature or less before administering. When infusing 20% or 25% mannitol concentrations, the administration set should include a filter.
Protect from freezing. Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
|
Revised: June, 2009 |
||
|
Printed in USA |
EN-2164 |
Hospira, Inc., Lake Forest, IL 60045 USA
Cardinal Health
Zanesville, OH 43701
IA66880711
Principal Display Panel
25% Mannitol Injection, USP
(12.5 g Total)
1 x 50 mL Single-Dose Fliptop Vial

MannitolMANNITOL INJECTION, SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||