mark. back up plan
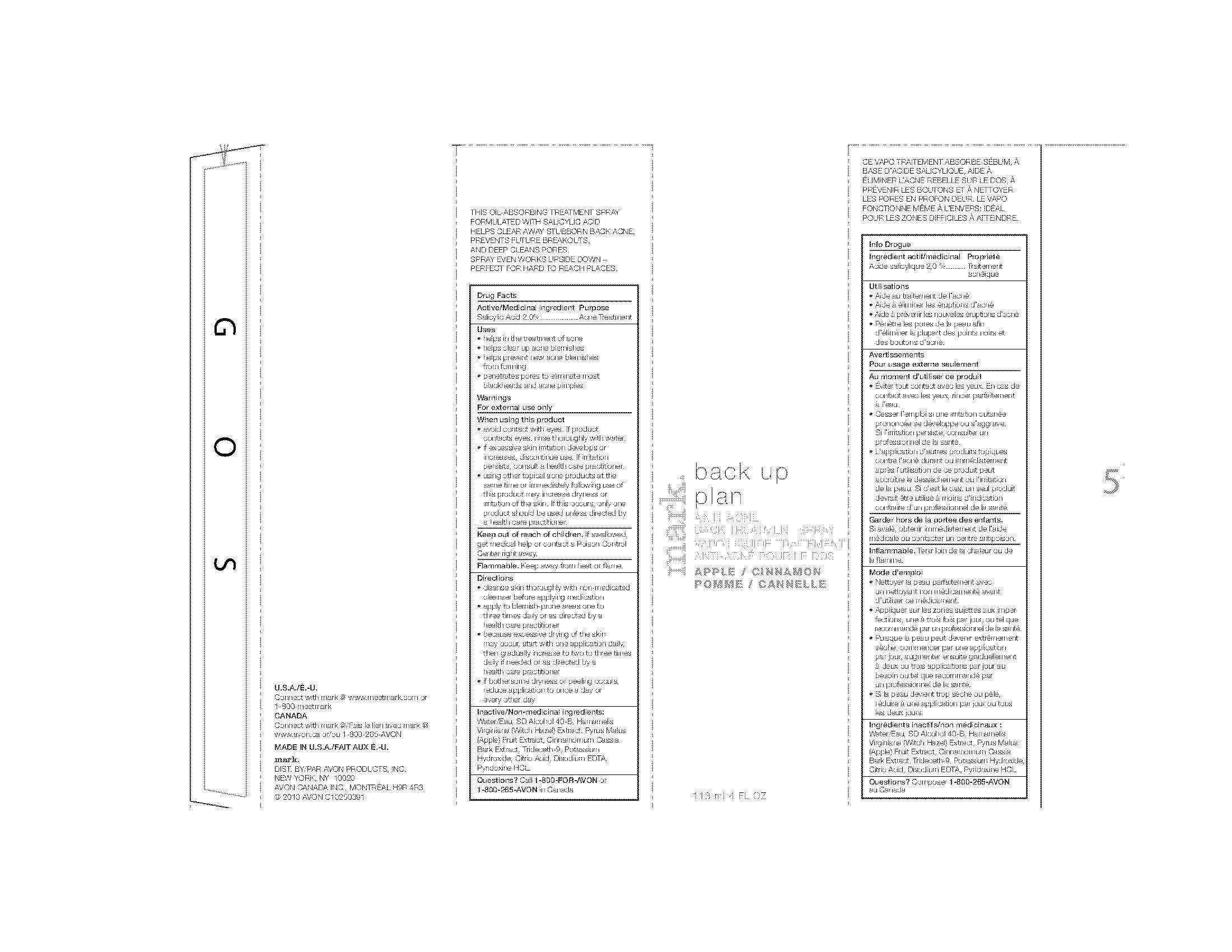
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Active/Medicinal ingredient PurposeSalicylic Acid 2.0%..................Acne Treatment
Uses
Uses
• helps in the treatment of acne
• helps clear up acne blemishes
• helps prevent new acne blemishes
from forming
• penetrates pores to eliminate most
blackheads and acne pimples
Warnings
For external use only
When using this product
• avoid contact with eyes. If product
contacts eyes, rinse thoroughly with water.
• if excessive skin irritation develops or
increases, discontinue use. If irritation
persists, consult a health care practitioner.
• using other topical acne products at the
same time or immediately following use of
this product may increase dryness or
irritation of the skin. If this occurs, only one
product should be used unless directed by
a health care practitioner
Keep out of reach of children. If swallowed,
get medical help or contact a Poison Control
Center right away.
Flammable. Keep away from heat or flame.
Directions
• cleanse skin thoroughly with non-medicated
cleanser before applying medication
• apply to blemish-prone areas one to
three times daily or as directed by a
health care practitioner
• because excessive drying of the skin
may occur, start with one application daily,
then gradually increase to two to three times
daily if needed or as directed by a
health care practitioner
• if bothersome dryness or peeling occurs,
reduce application to once a day or
every other day
Inactive/Non-medicinal ingredients:
Water/Eau, SD Alcohol 40-B, Hamamelis
Virginiana (Witch Hazel) Extract, Pyrus Malus
(Apple) Fruit Extract, Cinnamomum Cassia
Bark Extract, Trideceth-9, Potassium
Hydroxide, Citric Acid, Disodium EDTA,
Pyridoxine HCL.
Questions? Call 1-800-FOR-AVON or
1-800-265-AVON in Canada
mark. back up planSalicylic Acid SPRAY
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||