Marquis
Marquis(15% w/w ponazuril)
FULL PRESCRIBING INFORMATION: CONTENTS*
- Antiprotozoal Oral Paste
- MARQUIS DESCRIPTION:
- CHEMICAL NOMENCLATURE AND STRUCTURE:
- CLINICAL PHARMACOLOGY:
- INDICATIONS:
- EFFECTIVENESS SUMMARY:
- WARNING:
- PRECAUTIONS:
- MARQUIS ADVERSE REACTIONS:
- ANIMAL SAFETY SUMMARY:
- DOSAGE:
- HOW SUPPLIED:
- REFERENCES:
- PRINCIPAL DISPLAY PANELS:
FULL PRESCRIBING INFORMATION
Antiprotozoal Oral Paste
Caution: Federal (U.S.A.) Law restricts this drug to use by or on the order of a licensed veterinarian.
For The Treatment Of Equine
Protozoal Myeloencephalitis (EPM) In Horses
For Oral Use Only
DESCRIPTION:
Marquis® (15% w/w ponazuril) Antiprotozoal Oral Paste is supplied in ready-to-use syringes containing 127 grams of paste. Each gram of paste contains 150 mg of ponazuril (15% w/w). Marquis (ponazuril) is designed to be delivered as an orally administered paste.
Each syringe barrel of Marquis (ponazuril) contains enough paste to treat one (1) 1,200 lb (544 kg) horse for seven (7) days, at a dose rate of 5 mg/kg (2.27 mg/lb) body weight. The plunger contains a dosage ring calibrated for a dose rate of 5 mg/kg (2.27 mg/lb) body weight and marked for horse weight from 600 to 1,200 lbs (272 to 544 kg). The syringe barrels are packaged in units of four with four reusable plungers. This package provides sufficient paste to treat one 1,200 lb (544 kg) horse for 28 days at a dose rate of 5 mg/kg (2.27 mg/lb) body weight.
Ponazuril is an anticoccidial (antiprotozoal) compound with activity against several genera of the phylum Apicomplexa.
CHEMICAL NOMENCLATURE AND STRUCTURE:
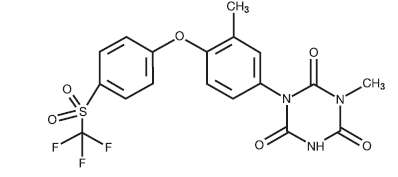
Ponazuril 1,3,5-Triazine-2,4,6(1H, 3H, 5H)-trione,1-methyl-3-[3-methyl-4-[4-[(trifluoromethyl)sulfonyl] phenoxy]phenyl]-(9CI)
CLINICAL PHARMACOLOGY:
The activity of ponazuril has been demonstrated in several Apicomplexans1-6. Lindsay, Dubey and Kennedy7 showed that the concentration of ponazuril necessary to kill Sarcocystis neurona in vitro was 0.1 to 1.0 µg/mL. Furr and Kennedy8 evaluated the pharmacokinetics of ponazuril in serum and CSF in normal horses treated daily at 5 mg/kg for 28 days. The time to peak serum concentration (Tmax) was 18.20 (±5.9) days and the maximum serum concentration (Cmax) was 5.59 (±0.92) µg/mL. The terminal elimination half-life for serum (calculated using Day 28 to 42 data) was 4.50 (±0.57) days. In CSF, Tmax was 15.40 (±7.9) days and Cmax was 0.21 (±0.072) µg/mL.
INDICATIONS:
Marquis (ponazuril) is indicated for the treatment of equine protozoal myeloencephalitis (EPM) caused by Sarcocystis neurona.
EFFECTIVENESS SUMMARY:
A field study was conducted at six sites with seven investigators across the United States.9 The study was conducted using historical controls. In this study, each animal’s response to treatment was compared to its pre-treatment values. The following standardized neurologic scale was used to grade the horses:
0 – Normal, no deficit detected
1 – Deficit just detected at normal gait
2 – Deficit easily detected and is exaggerated by backing, turning, swaying, loin pressure or neck extension
3 – Deficit very prominent on walking, turning, loin pressure or neck extension
4 – Stumbling, tripping and falling down spontaneously
5 – Recumbent, unable to rise
Improvement was defined as a decrease of at least one grade.
Naturally-occurring clinical cases of EPM, characterized by signalment and laboratory diagnosis, were randomly allotted to one of two treatment doses (5 or 10 mg/kg/day for a period of 28 days), then evaluated for clinical changes through 118 days. Acceptance into the study was based on the results from a standardized neurological examination including radiography, serum S. neurona IgG level determination by Western Blot (WB), and a positive cerebrospinal fluid (CSF) for S. neurona IgG level by WB.
Response to treatment was determined by the investigator to be acceptable when a clinical improvement of at least one grade occurred by no later than 3 months after treatment, regardless of whether the CSF by WB was positive or negative.
Changes in clinical condition were evaluated first by the subjective scoring of the investigator, then by masked assessment of videotapes of the neurological examination. At 5 mg/kg for 28 days, 28 of 47 horses (60%) improved at least one grade by Day 118. Seventy-five percent (75%) of those improved, that had also been videotaped, were corroborated successes by videotape assessment. At 10 mg/kg, 32 of 55 animals (58%) improved at least one grade by Day 118 and 56% of those improved, that had also been videotaped, were corroborated successes using videotape assessment. With respect to the clinical investigators’ scores there was no statistical difference between 5 mg/kg and 10 mg/kg treatment group results (p = 0.8867).
WARNING:
For use in animals only. Not for use in horses intended for human consumption. Not for human use. Keep out of the reach of children.
PRECAUTIONS:
Prior to treatment, EPM should be distinguished from other diseases that may cause ataxia in horses. Injuries or lameness may also complicate the evaluation of an animal with EPM. In most instances, ataxia due to EPM is asymmetrical and affects the hind limbs.
Clinicians should recognize that clearance of the parasite by ponazuril may not completely resolve the clinical signs attributed to the natural progression of the disease. The prognosis for animals treated for EPM may be dependent upon the severity of disease and the duration of the infection prior to treatment.
The safe use of Marquis (ponazuril) in horses used for breeding purposes, during pregnancy, or in lactating mares, has not been evaluated. The safety of Marquis (ponazuril) with concomitant therapies in horses has not been evaluated.
ADVERSE REACTIONS:
In the field study, eight animals were noted to have unusual daily observations. Two horses exhibited blisters on the nose and mouth at some point in the field study, three animals showed a skin rash or hives for up to 18 days, one animal had loose stools throughout the treatment period, one had a mild colic on one day and one animal had a seizure while on medication. The association of these reactions to treatment was not established.
ANIMAL SAFETY SUMMARY:
Marquis (ponazuril) was administered to 24 adult horses (12 males and 12 females) in a target animal safety study. Three groups of 8 horses each received 0, 10, or 30 mg/kg (water as control, 2X and 6X for a 5 mg/kg [2.27 mg/lb] dose). Horses were dosed after feeding. One half of each group was treated for 28 days and the other half for 56 days followed by necropsy upon termination of treatment. There were several instances of loose feces in all animals in the study irrespective of treatment, sporadic inappetence and one horse at 10 mg/kg (2X) lost weight while on test. Loose feces were treatment related. Histopathological findings included moderate edema in the uterine epithelium of three of the four females in the 6X group (two treated for 28 days and one for 56 days).
For customer service or to obtain product information, including Material Safety Data Sheet, call 1-800-633-3796. For medical emergencies or to report adverse reactions, call 1-800-422-9874.
DOSAGE:
Marquis (15% w/w ponazuril) is to be used at a dose of 5 mg/kg (2.27 mg/lb) body weight once daily for a period of 28 days.
ADMINISTRATION:
Paste syringe assembly:
Before administration, the syringe barrel and plunger require assembly. Ensure plunger is clean and dry.
Step 1. End cap must be on syringe barrel when inserting plunger.

Step 2. Carefully insert reusable plunger into base of syringe barrel until it snaps into place, then remove end cap and gently apply pressure to the plunger until paste is seen at the tip of the syringe barrel.
Step 3. Return end cap to tip of paste syringe.
Administering Marquis (ponazuril) to the horse:
NOTE: The paste syringe is a multi-dose package. Ensure that the correct dose is administered with each use.
Step 1. Remove end cap and gently apply pressure to the plunger until paste is seen at the tip of the syringe barrel. Return end cap to tip of paste syringe.

Step 2. Determine weight of horse and insure the horse’s mouth contains no feed.

Step 3. To measure dose, dosage ring collar and barrel collar should be flush. Hold plunger and rotate dosage ring with the other hand to the weight of the horse.
Step 4. Remove end cap from tip of syringe barrel.
Step 5. The selected dose of paste should be deposited onto the back and top of the horse’s tongue. Introduce tip of paste syringe into the side of the horse’s mouth at the space between the front (incisor) and back (molar) teeth. Deposit paste on the horse’s tongue by depressing the plunger of the syringe as far as the dose ring permits. Remove tip of syringe from horse’s mouth.

Step 6. To aid swallowing of paste, immediately raise horse’s head for a few seconds after dosing.

Step 7. Clean the tip of the syringe with a clean disposable towel and return end cap to tip of syringe barrel.
Step 8. For the next daily dose, repeat steps 1-7.
NOTE: At the end of the prescribed treatment period, partially used syringes should be discarded.
Store at Controlled Room Temperature 15-30º C (59-86º F).
HOW SUPPLIED:
Code: 08711553-045799 Carton contains four (4) x 127 gram syringe applicators and four (4) reusable syringe plungers
REFERENCES:
1. Mehlhorn, H., Ortmann-Falkenstein, G., Haberkorn, A.: (1984) The effects of the sym. Trianzinons on developmental stages of Eimeria tenella, E. maxima and E. acervulina: a light and electron microscopical study. Zeitschr Parsitenk 70: 173-182.
2. Bohrmann, R.: (1991) Treatment with toltrazuril in a natural outbreak of coccidiosis in calves. Dtsch. Tierarzl. Wschr. 98: 343-345.
3. Stafford, K.J., West, D.M., Vermunt, J.J., Pomroy, W., Adlington, B.A., Calder, S.M.: (1994) The effect of repeated doses of toltrazuril on coccidial oocyst output and weight gain in infected lambs. NZ Vet J 42(3): 117-119.
4. Haberkorn, A.G., Stoltefuss, D.I.J.: (1987) Studies on the activity spectrum of toltrazuril, a new anti-coccidial compound. Vet. Med. Review 1: 22-32.
5. Koudela, B., Vodstricilova, M., Klimes, B., Vladik, P., Vitovec, J.: (1991) Application of the anticoccidiosis drug Toltrazuril in the coccidiosis of neonatal pigs. Veterinami medicina (Praha) 36: 657-663.
6. Benoit, E., Buronfosse, T., Delatour, P.: (1994) Effect of Cytochrome P-450 1A induction on enantioselective metabolism and pharmacokinetics of an aryltrifluoromethyl sulfide in the rat. Chirality 6(5): 372-377.
7. Lindsay, D.S., Dubey, J.P., Kennedy, T.J.: (2000) Determination of the activity of ponazuril against Sarcocystis neurona in cell cultures. Vet Parasit 92: 165-169.
8. Furr, M., Kennedy, T.: Pharmacokinetics of ponazuril in horses. Study 150-717 Bayer Corporation.
9. Furr, M., Andrews, F., MacKay, R., Reed, S., Bernard, W., Bain, F., Byars, D., Kennedy, T.: Treatment of equine protozoal myeloencephalitis (EPM) with various doses of ponazuril. Study Report 150-664 Bayer Corporation.
U.S. Patent No. 5,883,095
©2012 Bayer HealthCare LLC
Manufactured by Bayer HealthCare LLC
Animal Health Division
Shawnee Mission, Kansas 66201 U.S.A.
www.bayerdvm.com
NADA #141-188, Approved by FDA
Bayer (reg’d), the Bayer Cross (reg’d), Marquis®, and the horse logo are trademarks of Bayer.
08715532-79004570, R.4
July, 2012
PRINCIPAL DISPLAY PANELS:


Marquisponazuril PASTE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||