Meclizine Hydrochloride
Cardinal Health
UDL Laboratories, Inc.
FULL PRESCRIBING INFORMATION: CONTENTS*
- MECLIZINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- MECLIZINE HYDROCHLORIDE INDICATIONS AND USAGE
- MECLIZINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- MECLIZINE HYDROCHLORIDE ADVERSE REACTIONS
- MECLIZINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED:
- Principal Display Panel - 12.5 mg Bag
- Principal Display Panel - 25 mg Bag
FULL PRESCRIBING INFORMATION
MECLIZINE HYDROCHLORIDE DESCRIPTION
Meclizine hydrochloride, an oral antiemetic, is a white, slightly yellowish, crystalline powder which has a slight odor and is tasteless. It has the following structural formula:
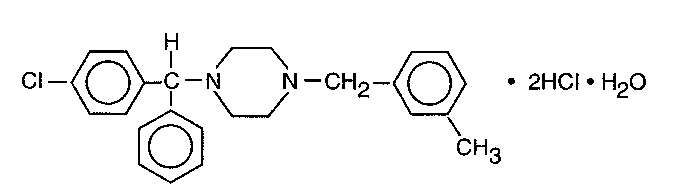
C25H27CIN2•2HCI•H2O M.W. 481.89
The chemical name is 1-(p-chloro-alpha-phenylbenzyl)-4-(m-methyl-benzyl) - piperazine dihydrochloride monohydrate.
Meclizine Hydrochloride Tablets are available in 12.5 mg, and *25 mg strengths for oral administration.
*Contains FD&C Yellow #5 (see PRECAUTIONS).
Each tablet contains the following inactive ingredients: colloidal silicon dioxide, lactose, magnesium stearate, microcrystalline cellulose, sodium starch glycolate, starch, and stearic acid. In addition, the 12.5 mg tablet contains FD&C Blue #1; and the 25 mg tablet contains D&C Yellow #10 and FD&C Yellow #5.
CLINICAL PHARMACOLOGY
Meclizine hydrochloride is an antihistamine which shows marked protective activity against nebulized histamine and lethal doses of intravenously injected histamine in guinea pigs. It has a marked effect in blocking the vasodepressor response to histamine, but only a slight blocking action against acetylcholine. Its activity is relatively weak in inhibiting the spasmogenic action of histamine on isolated guinea pig ileum.
MECLIZINE HYDROCHLORIDE INDICATIONS AND USAGE
For the prevention and treatment of nausea, vomiting, or dizziness associated with motion sickness.
MECLIZINE HYDROCHLORIDE CONTRAINDICATIONS
Meclizine hydrochloride is contraindicated in individuals who have shown a previous hypersensitivity to it.
WARNINGS
Since drowsiness may, on occasion, occur with use of this drug, patients should be warned of this possibility and cautioned against driving a car or operating dangerous machinery.
Patients should avoid alcoholic beverages while taking the drug. Due to its potential anticholinergic action, this drug should be used with caution in patients with asthma, glaucoma, or enlargement of the prostate gland. Do not give to children under 12 years of age unless directed by a doctor.
PRECAUTIONS
The Meclizine Hydrochloride Tablets, 25 mg contain FD&C Yellow #5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible individuals. Although the overall incidence of FD&C Yellow #5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
Usage in Children: Clinical studies establishing safety and effectiveness in children have not been done; therefore, usage is not recommended under 12 years of age.
Usage in Pregnancy: Pregnancy Category B. Reproduction studies in rats have shown cleft palates at 25-50 times the human dose. Epidemiological studies in pregnant women, however, do not indicate that meclizine hydrochloride increases the risk of abnormalities when administered during pregnancy.
Despite the animal findings, it would appear that the possibility of fetal harm is remote. Nevertheless, meclizine hydrochloride, or any other medication should be used during pregnancy only if clearly necessary.
MECLIZINE HYDROCHLORIDE ADVERSE REACTIONS
Drowsiness, dry mouth, and on rare occasions, blurred vision have been reported.
MECLIZINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
Motion Sickness: The initial dose of 25 to 50 mg meclizine hydrochloride, should be taken one hour prior to travel for protection against motion sickness. Thereafter, the dose may be repeated every 24 hours for the duration of the journey.
HOW SUPPLIED:
Meclizine Hydrochloride Tablets, USP 12.5 mg - blue, oval tablets debossed with “034” on one side and “par” on the other side. Tablets may contain characteristic dye spots. They are available as follows:
NDC 51079-089-20 - Unit dose blister packages of 100 (10 cards of 10 tablets each).
Meclizine Hydrochloride Tablets, USP 25 mg - yellow, oval tablets debossed with “035” on one side and “par” on the other side. They are available as follows:
NDC 51079-090-20 - Unit dose blister packages of 100 (10 cards of 10 tablets each).
Store at controlled room temperature 15° to 30°C (59° to 86°F).
Manufactured by:
Par Pharmaceutical Companies, Inc.
Spring Valley, NY 10977
Distributed by:
UDL Laboratories, Inc.
Rockford, IL 61103
S-5278 R13
3/10
Principal Display Panel - 12.5 mg Bag
Meclizine Hydrochloride Tablets, USP
12.5 mg
10 Tablets

Principal Display Panel - 25 mg Bag
Meclizine Hydrochloride Tablets, USP
25 mg
10 Tablets

Meclizine Hydrochloridemeclizine hydrochloride TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Meclizine Hydrochloridemeclizine hydrochloride TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||