Mederma
Mederma CREAM
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
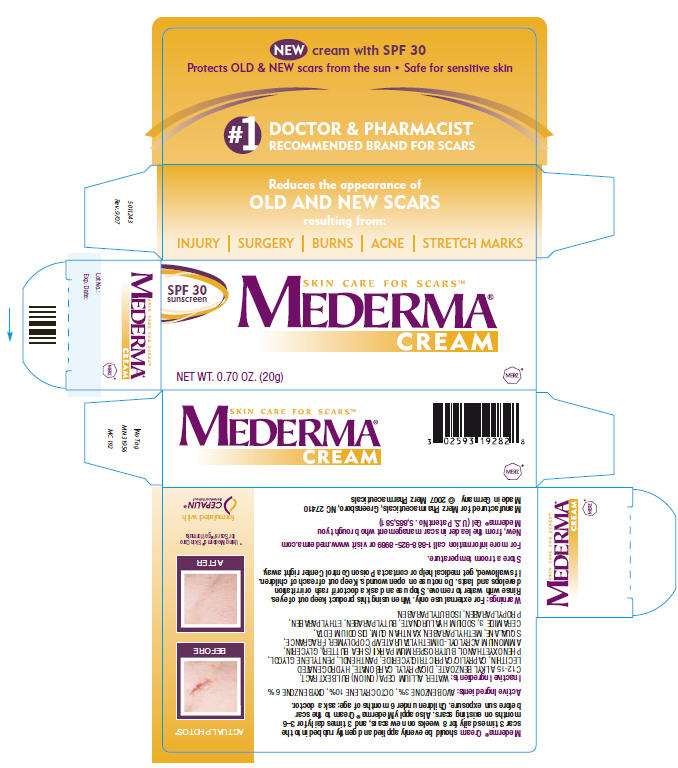
Mederma® Cream should be evenly applied and gently rubbed into the scar 3 times daily for 8 weeks on new scars, and 3 times daily for 3–6 months on existing scars. Also apply Mederma® Cream to the scar before sun exposure.
Children under 6 months of age: ask a doctor.
Active Ingredients
AVOBENZONE 3%, OCTOCRYLENE 10%, OXYBENZONE 6%
Inactive Ingredients
WATER, ALLIUM CEPA (ONION) BULB EXTRACT, C12-15 ALKYL BENZOATE, DICAPRYLYL CARBONATE, HYDROGENATED LECITHIN, CAPRYLIC/CAPRIC TRIGLYCERIDE, PANTHENOL, PENTYLENE GLYCOL, PHENOXYETHANOL, BUTYROSPERMUM PARKII (SHEA BUTTER), GLYCERIN, AMMONIUM ACRYLOYL-DIMETHYLTAURATE/VP COPOLYMER, FRAGRANCE, SQUALANE, METHYLPARABEN, XANTHAN GUM, DISODIUM EDTA, CERAMIDE 3, SODIUM HYALURONATE, BUTYLPARABEN, ETHYLPARABEN, PROPYLPARABEN, ISOBUTYLPARABEN.
Warnings
For external use only.
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash or irritation develops and lasts.
Do not use on open wounds.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Store at room temperature.
For more information call 1-888-925-8989 or visit www.mederma.com
Manufactured for Merz Pharmaceuticals, Greensboro, NC 27410
Made in Germany © 2007 Merz Pharmaceuticals
PRINCIPAL DISPLAY PANEL - 20g Carton
SPF 30
sunscreen
SKIN CARE FOR SCARS™
MEDERMA®
CREAM
NET WT 0.70 OZ. (20g)
MERZ®
MedermaAVOBENZONE, OCTOCRYLENE, and OXYBENZONE CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||