Medrol
FULL PRESCRIBING INFORMATION: CONTENTS*
- MEDROL DESCRIPTION
- INDICATIONS & USAGE
- MEDROL CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DRUG INTERACTIONS
- INFORMATION FOR PATIENTS
- MEDROL ADVERSE REACTIONS
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
MEDROL DESCRIPTION
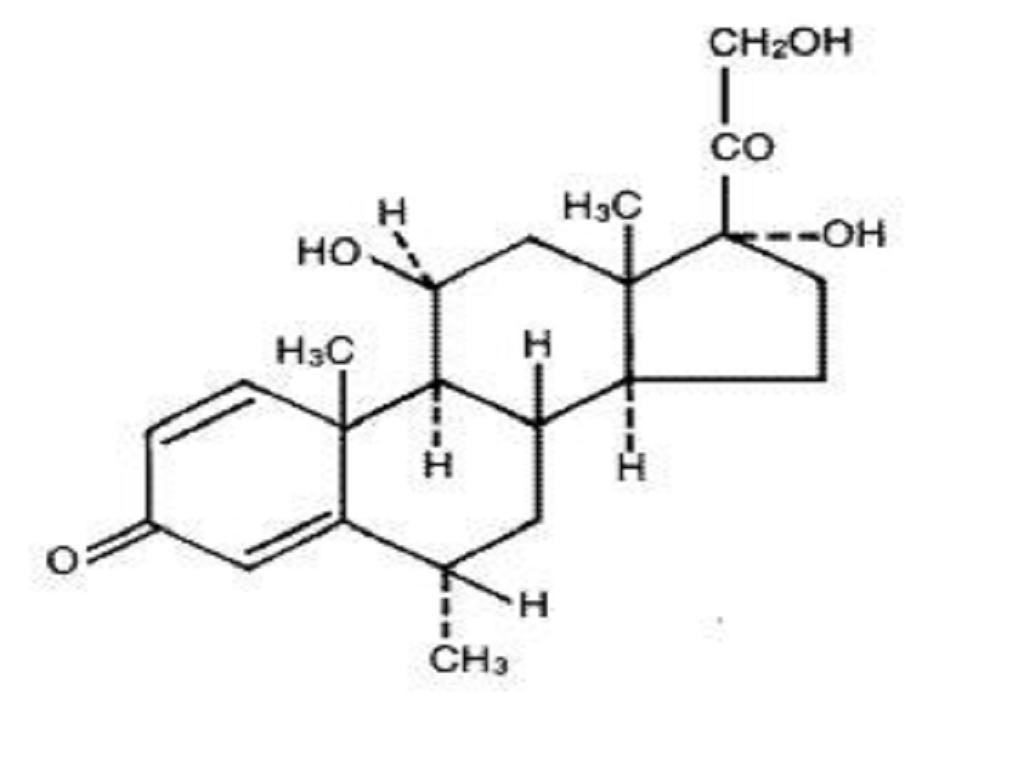
ACTIONS
INDICATIONS & USAGE
1. Endocrine Disorders
2. Rheumatic Disorders
3. Collagen Diseases
4. Dermatologic Diseases
5. Allergic States
6. Ophthalmic Diseases
7. Respiratory Diseases
8. Hematologic Disorders
9. Neoplastic Diseases
10. Edematous States
11. Gastrointestinal Diseases
12. Nervous System
13. Miscellaneous
MEDROL CONTRAINDICATIONS
WARNINGS
Usage in pregnancy
PRECAUTIONS
General PrecautionsDOSAGE AND ADMINISTRATION
DRUG INTERACTIONS
INFORMATION FOR PATIENTS
MEDROL ADVERSE REACTIONS
-
● Sodium retention
-
● Congestive heart failure in susceptible patients
-
● Hypertension
-
● Fluid retention
-
● Potassium loss
-
● Hypokalemic alkalosis
-
● Muscle weakness
-
● Loss of muscle mass
-
● Steroid myopathy
-
● Osteoporosis
-
● Tendon rupture, particularly of the Achilles tendon
-
● Vertebral compression fractures
-
● Aseptic necrosis of femoral and humeral heads
-
● Pathologic fracture of long bones
-
● Peptic ulcer with possible perforation and hemorrhage
-
● Pancreatitis
-
● Abdominal distention
-
● Ulcerative esophagitis
-
● Impaired wound healing
-
● Petechiae and ecchymoses
-
● May suppress reactions to skin tests
-
● Thin fragile skin
-
● Facial erythema
-
● Increased sweating
-
● Increased intracranial pressure with papilledema (pseudo-tumor cerebri) usually after treatment
-
● Convulsions
-
● Vertigo
-
● Headache
-
● Development of Cushingoid state
-
● Suppression of growth in children
-
● Secondary adrenocortical and pituitary unresponsiveness, particularly in times of stress, as in trauma, surgery or illness
-
● Menstrual irregularities
-
● Decreased carbohydrate tolerance
-
● Manifestations of latent diabetes mellitus
-
● Increased requirements of insulin or oral hypoglycemic agents in diabetics
-
● Posterior subcapsular cataracts
-
● Increased intraocular pressure
-
● Glaucoma
-
● Exophthalmos
-
● Negative nitrogen balance due to protein catabolism
DOSAGE & ADMINISTRATION
IT SHOULD BE EMPHASIZED THAT DOSAGE REQUIREMENTS ARE VARIABLE AND MUST BE INDIVIDUALIZED ON THE BASIS OF THE DISEASE UNDER TREATMENT AND THE RESPONSE OF THE PATIENT.
Multiple Sclerosis
ADT(Alternate Day Therapy)
HOW SUPPLIED
2 mg
4 mg
8 mg
16 mg
32 mg
STORAGE AND HANDLING
REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

MedrolMethylprednisolone TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!