Meloxicam
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- MELOXICAM DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- USE IN SPECIFIC POPULATIONS
- INDICATIONS & USAGE
- MELOXICAM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- MELOXICAM ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- INFORMATION FOR PATIENTS
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
Cardiovascular Risk-
● NSAIDs may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increase with duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at greater risk (seeWARNINGSandCLINICAL TRIALS).
-
● Meloxicam is contraindicated for the treatment of perioperative pain in the setting of coronary artery bypass graft (CABG) surgery (seeWARNINGS).
-
● NSAIDs cause an increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events (seeWARNINGS).
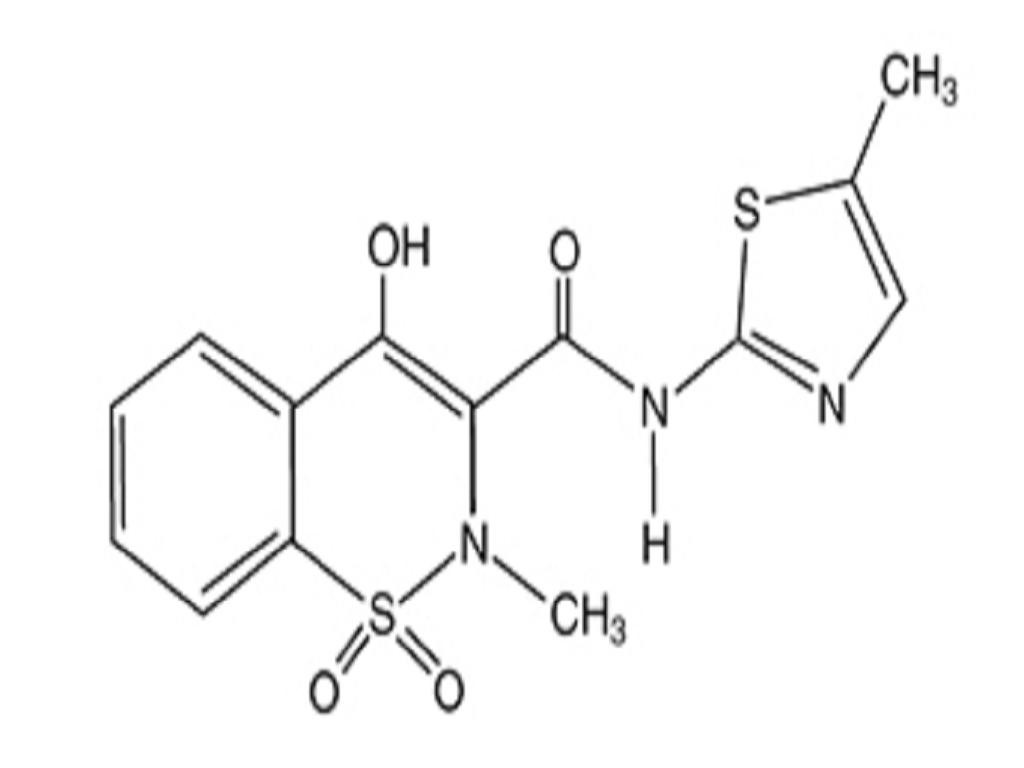
MELOXICAM DESCRIPTION
CLINICAL PHARMACOLOGY
Mechanism of ActionPHARMACOKINETICS
Absorption*
*
Food and Antacid Effects
Distribution
Metabolism
Excretion
USE IN SPECIFIC POPULATIONS
GeriatricGender
Hepatic Insufficiency
Renal Insufficiency
WARNINGS: Advanced Renal Disease
Hemodialysis
CLINICAL TRIALS
Osteoarthritis and Rheumatoid Arthritis
The use of meloxicam for the treatment of the signs and symptoms of osteoarthritis of the knee and hip was evaluated in a 12 week double-blind controlled trial. Meloxicam (3.75 mg, 7.5 mg and 15 mg daily) was compared to placebo. The four primary endpoints were investigator's global assessment, patient global assessment, patient pain assessment, and total WOMAC score (a self-administered questionnaire addressing pain, function and stiffness). Patients on meloxicam 7.5 mg daily and meloxicam 15 mg daily showed significant improvement in each of these endpoints compared with placebo.
The use of meloxicam for the management of signs and symptoms of osteoarthritis was evaluated in six double-blind, active-controlled trials outside the U.S. ranging from 4 weeks to 6 months duration. In these trials, the efficacy of meloxicam, in doses of 7.5 mg/day and 15 mg/day, was comparable to piroxicam 20 mg/day and diclofenac SR 100 mg/day and consistent with the efficacy seen in the U.S. trial.
The use of meloxicam for the treatment of the signs and symptoms of rheumatoid arthritis was evaluated in a 12-week double-blind, controlled multinational trial. Meloxicam (7.5 mg, 15 mg and 22.5 mg daily) was compared to placebo. The primary endpoint in this study was the ACR20 response rate, a composite measure of clinical, laboratory and functional measures of RA response. Patients receiving meloxicam 7.5 mg and 15 mg daily showed significant improvement in the primary endpoint compared with placebo. No incremental benefit was observed with the 22.5 mg dose compared to the 15 mg dose.
Higher doses of meloxicam (22.5 mg and greater) have been associated with an increased risk of serious GI events; therefore the daily dose of meloxicam should not exceed 15 mg.
INDICATIONS & USAGE
WARNINGSMELOXICAM CONTRAINDICATIONS
WARNINGS: Anaphylactoid ReactionsPRECAUTIONS: Preexisting Asthma
WARNINGS
WARNINGS
Cardiovascular EffectsCardiovascular Thrombotic Events
WARNINGS: Gastrointestinal (GI) Effects: Risk of GI Ulceration, Bleeding, and Perforation
CONTRAINDICATIONS
Hypertension
Congestive Heart Failure and Edema
Gastrointestinal (GI) Effects
Risk of GI Ulceration, Bleeding, and Perforation
Renal Effects
Advanced Renal Disease
Anaphylactoid Reactions
CONTRAINDICATIONSPRECAUTIONS: Preexisting Asthma
Skin Reactions
Pregnancy
PRECAUTIONS
GeneralHepatic Effects
Renal Effects
WARNINGS: Renal EffectsAdvanced Renal Disease
Hematological Effects
Preexisting Asthma
INFORMATION FOR PATIENTS
WARNINGS: Cardiovascular Effects
WARNINGS: Gastrointestinal (GI) Effects: Risk of GI Ulceration, Bleeding, and Perforation
WARNINGS
LABORATORY TESTS
DRUG INTERACTIONS
ACE InhibitorsAspirin
Cholestyramine
Cimetidine
Digoxin
Furosemide
WARNINGS: Renal Effects
Lithium
Methotrexate
Warfarin
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic EffectsPregnancy Category C
Nonteratogenic Effects
LABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
MELOXICAM ADVERSE REACTIONS
AdultsOsteoarthritis and Rheumatoid Arthritis
**
**
**
OVERDOSAGE
DOSAGE & ADMINISTRATION
Osteoarthritis and Rheumatoid ArthritisWARNINGS
HOW SUPPLIED
STORAGE AND HANDLING
INFORMATION FOR PATIENTS
Medication Guide
Cardiovascular Effects
5.1
Gastrointestinal Effects
5.2
Hepatotoxicity
5.3
Adverse Skin Reactions
5.8
Weight Gain and Edema
5.5
Anaphylactoid Reactions
5.7
Effects During Pregnancy
5.98.1
SPL MEDGUIDE
-
● with longer use of NSAID medicines
-
● in people who have heart disease
-
● can happen without warning symptoms
-
● may cause death
-
● taking medicines called "corticosteroids" and "anticoagulants"
-
● longer use
-
● smoking
-
● drinking alcohol
-
● older age
-
● having poor health
-
● exactly as prescribed
-
● at the lowest dose possible for your treatment
-
● for the shortest time needed
-
● different types of arthritis
-
● menstrual cramps and other types of short-term pain
-
● if you had an asthma attack, hives, or other allergic reaction with aspirin or any other NSAID medicine
-
● for pain right before or after heart bypass surgery
-
● about all of your medical conditions.
-
● about all of the medicines you take. NSAIDs and some other medicines can interact with each other and cause seri- ous side effects. Keep a list of your medicines to show to your healthcare provider and pharmacist.
-
● if you are pregnant. NSAID medicines should not be used by pregnant women late in their pregnancy.
-
● if you are breastfeeding. Talk to your doctor.
-
● shortness of breath or trouble breathing
-
● chest pain
-
● weakness in one part or side of your body
-
● slurred speech
-
● swelling of the face or throat
-
● nausea
-
● more tired or weaker than usual
-
● itching
-
● your skin or eyes look yellow
-
● stomach pain
-
● flu-like symptoms
-
● vomit blood
-
● there is blood in your bowel movement or it is black and sticky like tar
-
● skin rash or blisters with fever
-
● unusual weight gain
-
● swelling of the arms and legs, hands and feet
-
● Aspirin is an NSAID medicine but it does not increase the chance of a heart attack. Aspirin can cause bleeding in the brain, stomach, and intestines. Aspirin can also cause ulcers in the stomach and intestines.
-
● Some of these NSAID medicines are sold in lower doses without a prescription (over-theTalk to your healthcare provider before using over-the-counter NSAIDs for more than 10 days.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

MeloxicamMeloxicam TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!