Menopause
Menopause HP
FULL PRESCRIBING INFORMATION
Active ingredient
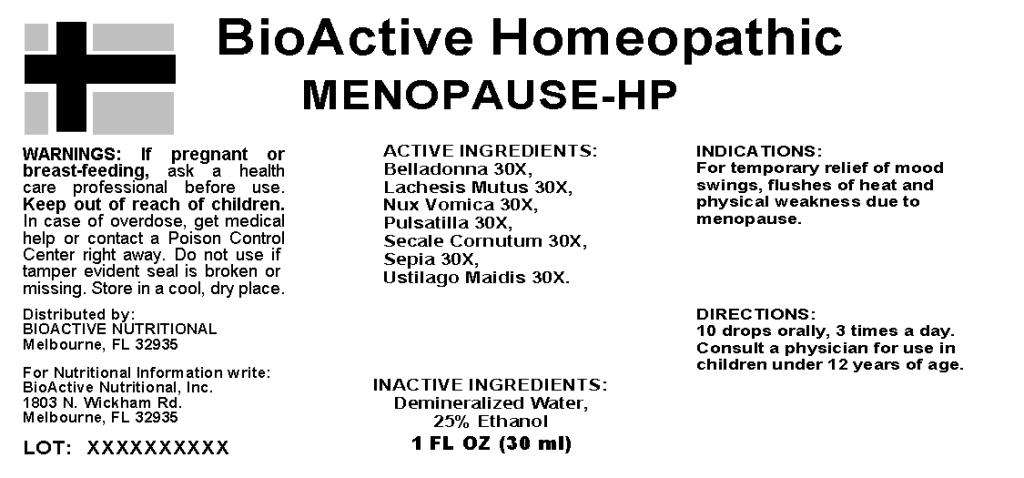
ACTIVE INGREDIENTS: Belladonna 30X, Lachesis mutus 30X, Nux vomica 30X, Pulsatilla 30X, Secale cornutum 30X, Sepia 30X, Ustilago maidis 30X.
Purpose
INDICATIONS: For temporary relief of mood swings, flushes of heat and physical weakness due to menopause.
WARNINGS: If pregnant or breast-feeding, ask a health care professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Do not use if tamper evident seal is broken or missing. Store in a cool, dry place.
DIRECTIONS: 10 drops orally, 3 times a day. Consult a physician for use in children under 12 years of age.
INACTIVE INGREDIENTS: Demineralized water, 25% Ethanol.
KEEP OUT OF REACH OF CHILDREN. In case of overdose, get medical help or contact a Poison Control Center right away.
Uses
INDICATIONS: For temporary relief of mood swings, flushes of heat and physical weakness due to menopause.
Distributed by:
BIOACTIVE NUTRITIONAL
Melbourne, FL 32935
For Nutritional Information write:
BioActive Nutritional, Inc.
1803 N. Wickham Rd.
Melbourne, FL 32935
BioActive Homeopathic
MENOPAUSE HP
1 FL OX (30 ml)
MenopauseBelladonna, Lachesis mutus, Nux vomica, Pulsatilla, Secale cornutum, Sepia, Ustilago maidis, LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||