Meperidine Hydrochloride
MEPERIDINEHydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- MEPERIDINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- MEPERIDINE HYDROCHLORIDE INDICATIONS AND USAGE
- MEPERIDINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- MEPERIDINE HYDROCHLORIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- MEPERIDINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- RL-2386
FULL PRESCRIBING INFORMATION
Injection, USP
CII
Rx only
300 mg (10 mg/mL)
ONLY FOR USE WITH A COMPATIBLE
HOSPIRA PCA PUMP SET WITH INJECTOR
AND A COMPATIBLE HOSPIRA INFUSION
DEVICE.
MEPERIDINE HYDROCHLORIDE DESCRIPTION
Meperidine Hydrochloride Injection, USP 10 mg/mL is a sterile, nonpyrogenic, hypotonic solution of meperidine hydrochloride, USP, in an acetate buffer. This product is to be administered by the intravenous route via a compatible Hospira infusion device.
Each mL contains meperidine hydrochloride 10 mg. Sodium acetate, anhydrous 1.5 mg and glacial acetic acid, 0.0012 mL are added as buffers. pH 4.5 (3.5 to 6.0).
The solution contains no bacteriostat or antimicrobial agent and is intended only for use as a single-dose unit to provide analgesia via the intravenous route using a compatible Hospira infusion device.
Meperidine is classified pharmacologically as a synthetic narcotic analgesic.
Meperidine Hydrochloride is ethyl-1-methyl-4-phenylisonipecotate hydrochloride, a white, crystalline substance with a melting point of 186° to 189°C. It is readily soluble in water and has a neutral reaction and a slightly bitter taste. The solution is not decomposed by a short period of boiling.
It has the following structural formula:
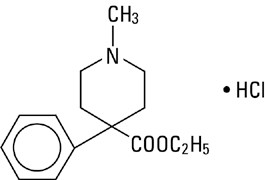
C15H21NO2 • HCl M.W. 283.80
CLINICAL PHARMACOLOGY
Meperidine hydrochloride is a narcotic analgesic with multiple actions qualitatively similar to those of morphine; the most prominent of these involve the central nervous system and organs composed of smooth muscle. The principal actions of therapeutic value are analgesia and sedation.
There is some evidence which suggests that meperidine may produce less smooth muscle spasm, constipation, and depression of the cough reflex than equianalgesic doses of morphine. Meperidine, in 60 mg to 80 mg parenteral doses, is approximately equivalent in analgesic effect to 10 mg of morphine. The onset of action is slightly more rapid than with morphine, and the duration of action is slightly shorter. Meperidine is significantly less effective by the oral than by the parenteral route, but the exact ratio of oral to parenteral effectiveness is unknown.
Meperidine is metabolized through biotransformation. The elimination half-life is 3 to 8 hours in healthy volunteers and is 1.3 to 2 times greater in post-operative or cirrhotic patients. The only bioactive metabolite is normeperidine which has an average elimination half-life of 20.6 hours. Elevated serum levels have been reported to cause central nervous system excitatory effects.
MEPERIDINE HYDROCHLORIDE INDICATIONS AND USAGE
Meperidine hydrochloride administered by slow intravenous injection is indicated for the relief of moderate to severe pain.
MEPERIDINE HYDROCHLORIDE CONTRAINDICATIONS
Hypersensitivity to meperidine.
Meperidine is contraindicated in patients who are receiving monoamine oxidase (MAO) inhibitors or those who have recently received such agents. Therapeutic doses of meperidine have occasionally precipitated unpredictable, severe, and occasionally fatal reactions in patients who have received such agents within 14 days. The mechanism of these reactions is unclear, but may be related to a pre-existing hyperphenylalaninemia. Some have been characterized by coma, severe respiratory depression, cyanosis, and hypotension, and have resembled the syndrome of acute narcotic overdose. In other reactions the predominant manifestations have been hyperexcitability, convulsions, tachycardia, hyperpyrexia, and hypertension. Although it is not known that other narcotics are free of the risk of such reactions, virtually all of the reported reactions have occurred with meperidine. If a narcotic is needed in such patients, a sensitivity test should be performed in which repeated, small, incremental doses of morphine are administered over the course of several hours while the patient’s condition and vital signs are under careful observation. (Intravenous hydrocortisone or prednisolone have been used to treat severe reactions, with the addition of intravenous chlorpromazine in those cases exhibiting hypertension and hyperpyrexia. The usefulness and safety of narcotic antagonists in the treatment of these reactions is unknown).
Solutions of meperidine hydrochloride and barbiturates are chemically incompatible.
WARNINGS
Drug Dependence. Meperidine can produce drug dependence of the morphine type and, therefore, has the potential for being abused. Psychic dependence, physical dependence, and tolerance may develop upon repeated administration of meperidine, and it should be prescribed and administered with the same degree of caution appropriate to the use of morphine. Like other narcotics, meperidine is subject to the provisions of the Federal narcotic laws.
Interaction with Other Central Nervous System Depressants. MEPERIDINE SHOULD BE USED WITH GREAT CAUTION AND IN REDUCED DOSAGE IN PATIENTS WHO ARE CONCURRENTLY RECEIVING OTHER NARCOTIC ANALGESICS, GENERAL ANESTHETICS, PHENOTHIAZINES, OTHER TRANQUILIZERS (SEE DOSAGE AND ADMINISTRATION), SEDATIVE-HYPNOTICS (INCLUDING BARBITURATES), TRICYCLIC ANTIDEPRESSANTS, AND OTHER CNS DEPRESSANTS (INCLUDING ALCOHOL). RESPIRATORY DEPRESSION, HYPOTENSION, AND PROFOUND SEDATION OR COMA MAY RESULT.
Head Injury and Increased Intracranial Pressure. The respiratory depressant effects of meperidine and its capacity to elevate cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions, or a preexisting increase in intracranial pressure. Furthermore, narcotics produce adverse reactions which may obscure the clinical course of patients with head injuries. In such patients, meperidine must be used with extreme caution and only if its use is deemed essential.
Intravenous Use: See DOSAGE AND ADMINISTRATION.
Asthma and Other Respiratory Conditions. Meperidine should be used with extreme caution in patients having an acute asthmatic attack, patients with chronic obstructive pulmonary disease or cor pulmonale, patients having a substantially decreased respiratory reserve, and patients with pre-existing respiratory depression, hypoxia, or hypercapnia. In such patients, even usual therapeutic doses of narcotics may decrease respiratory drive while simultaneously increasing airway resistance to the point of apnea.
Hypotensive Effect. The administration of meperidine may result in severe hypotension in the postoperative patient or any individual whose ability to maintain blood pressure has been compromised by a depleted blood volume or the administration of drugs such as the phenothiazines or certain anesthetics.
Usage in Ambulatory Patients. Meperidine may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery. The patient should be cautioned accordingly.
Meperidine, like other narcotics, may produce orthostatic hypotension in ambulatory patients.
Usage in Pregnancy and Lactation. Meperidine should not be used in pregnant women prior to the labor period, unless in the judgment of the physician the potential benefits outweigh the possible hazards, because safe use in pregnancy prior to labor has not been established relative to possible adverse effects on fetal development.
When used as an obstetrical analgesic, meperidine crosses the placental barrier and can produce depression of respiration and psychophysiologic functions in the newborn. Resuscitation may be required (see section on OVERDOSAGE).
Meperidine appears in the milk of nursing mothers receiving the drug.
PRECAUTIONS
Do not use unless solution is clear and package is undamaged. (See DOSAGE AND ADMINISTRATION.)
General:
Supraventricular Tachycardias. Meperidine should be used with caution in patients with atrial flutter and other supraventricular tachycardias because of a possible vagolytic action which may produce a significant increase in the ventricular response rate.
Convulsions. Meperidine may aggravate pre-existing convulsions in patients with convulsive disorders. If dosage is escalated substantially above recommended levels because of tolerance development, convulsions may occur in individuals without a history of convulsive disorders. The convulsive potential of meperidine may be further increased if prolonged infusions or repeated doses are administered due to high serum levels of normeperidine.
Acute Abdominal Conditions. The administration of meperidine or other narcotics may obscure the diagnosis or clinical course in patients with acute abdominal conditions.
Special Risk Patients. Meperidine should be given with caution and the initial dose should be reduced in certain patients such as the elderly or debilitated, and those with severe impairment of hepatic or renal function, hypothyroidism, Addison’s disease, and prostatic hypertrophy or urethral stricture.
Information for Patients:
Physicians should assure the patient, or their caregiver, has received adequate instructions for use prior to commencing therapy via PCA.
Pediatric Use. Meperidine Hydrochloride administered by the intravenous route via a compatible infusion device is not recommended for use in individuals younger than 19 years of age.
MEPERIDINE HYDROCHLORIDE ADVERSE REACTIONS
The major hazards of meperidine, as with other narcotic analgesics, are respiratory depression and, to a lesser degree, circulatory depression; respiratory arrest, shock, and cardiac arrest have occurred.
The most frequently observed adverse reactions include light-headedness, dizziness, sedation, nausea, vomiting, and sweating. These effects seem to be more prominent in ambulatory patients and in those who are not experiencing severe pain. In such individuals, lower doses are advisable. Some adverse reactions in ambulatory patients may be alleviated if the patient lies down. Other adverse reactions include:
Nervous System. Euphoria, dysphoria, weakness, headache, agitation, tremor, uncoordinated muscle movements, severe convulsions, transient hallucinations and disorientation, visual disturbances. Inadvertent injection about a nerve trunk may result in sensory-motor paralysis which is usually, though not always, transitory.
Gastrointestinal. Dry mouth, constipation, biliary tract spasm.
Cardiovascular. Flushing of the face, tachycardia, bradycardia, palpitation, hypotension (see WARNINGS), syncope, phlebitis following intravenous injection.
Genitourinary. Urinary retention.
Allergic. Pruritus, urticaria, other skin rashes, wheal and flare over the vein with intravenous injection.
Other. Pain at injection site; local tissue irritation and induration following subcutaneous injection, particularly when repeated; antidiuretic effect.
DRUG ABUSE AND DEPENDENCE
Meperidine is a Schedule II controlled substance.
Meperidine hydrochloride may cause psychological and physical dependence. (See WARNINGS.) Physical dependence results in withdrawal symptoms in patients who abruptly discontinue the drug or may be precipitated through the administration of a drug with narcotic antagonist activity, e.g., pentazocine. (See also OVERDOSAGE.) Severe narcotic abstinence syndrome is characterized by restlessness, lacrimation, rhinorrhea, yawning, perspiration, goose flesh, restless sleep or “yen” and mydriasis during the first 24 hours. These symptoms increase in severity and over the next 72 hours may be accompanied by increasing irritability, anxiety, weakness, twitching and spasm of muscles; kicking movements; severe backache, abdominal and leg pain; abdominal and muscle cramps; hot and cold flashes; insomnia; nausea, anorexia, vomiting, intestinal spasm, diarrhea; coryza and repetitive sneezing; increase in body temperature, blood pressure, respiratory rate and heart rate. Because of excessive loss of fluids through sweating, vomiting and diarrhea, there is usually marked weight loss, dehydration, ketosis and disturbances in acid-base balance. Cardiovascular collapse may occur. Without treatment, most observable symptoms disappear in 5 to 14 days; however, there appears to be a phase of secondary or chronic abstinence which may last for 2 to 6 months characterized by insomnia, irritability and muscular aches.
In the treatment of physical dependence on meperidine hydrochloride, the patient may be detoxified by gradual reduction of narcotic dosage. If abstinence symptoms become severe, the patient may be given methadone. Temporary administration of tranquilizers and sedatives may aid in reducing patient anxiety and narcotic craving. Gastrointestinal disturbances or dehydration should be treated accordingly.
A drug-dependent woman who is pregnant should undergo withdrawal treatment prior to delivery.
Infants born to narcotic-dependent mothers are often themselves physically dependent. Diagnosis begins with a maternal history of drug abuse; chromatographic analysis of the neonate’s urine will assist in the diagnosis. Symptoms usually occur over the first 4 days after birth and include irritability, high-pitched shrill cry, tremors, hyperactivity, vomiting, diarrhea, fever, sustained embracing reflex and seizures. Narcotics, barbiturates, phenothiazines or sedative-hypnotics have been used to treat withdrawal symptoms in addicted neonates.
OVERDOSAGE
Symptoms. Serious overdosage with meperidine is characterized by respiratory depression (a decrease in respiratory rate and/or tidal volume, Cheyne-Stokes respiration, cyanosis), extreme somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, and sometimes bradycardia and hypotension. In severe overdosage, particularly by the intravenous route, apnea, circulatory collapse, cardiac arrest, and death may occur.
Treatment. Primary attention should be given to the re-establishment of adequate respiratory exchange through provision of a patent airway and institution of assisted or controlled ventilation. The narcotic antagonist, naloxone hydrochloride, is a specific antidote against respiratory depression which may result from overdosage or unusual sensitivity to narcotics, including meperidine. Therefore, an appropriate dose of this antagonist should be administered, preferably by the intravenous route, simultaneously with efforts at respiratory resuscitation.
An antagonist should not be administered in the absence of clinically significant respiratory or cardiovascular depression.
Oxygen, intravenous fluids, vasopressors, and other supportive measures should be employed as indicated.
NOTE: In an individual physically dependent on narcotics, the administration of the usual dose of a narcotic antagonist will precipitate an acute withdrawal syndrome. The severity of this syndrome will depend on the degree of physical dependence and the dose of antagonist administered. The use of narcotic antagonists in such individuals should be avoided if possible. If a narcotic antagonist must be used to treat serious respiratory depression in the physically dependent patient, the antagonist should be administered with extreme care and only one-fifth to one-tenth the usual initial dose administered.
The use of naloxone in the presence of convulsive or other excitatory states and other manifestations of elevated normeperidine serum levels is not recommended. Most animal studies have shown that seizures due to normeperidine are not naloxone reversible.
MEPERIDINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
For use as a single-dose unit to provide analgesia via the intravenous route using a compatible Hospira infusion device. Each vial is intended for SINGLE DOSE ONLY. When the dosing requirement is complete, the unused portion should be discarded in an appropriate manner. DO NOT AUTOCLAVE.
PHYSICIANS SHOULD COMPLETELY FAMILIARIZE THEMSELVES WITH A COMPATIBLE HOSPIRA INFUSION DEVICE BEFORE DECIDING TO ADMINISTER MEPERIDINE HYDROCHLORIDE INJECTION VIA THE INFUSER.
Dosage should be adjusted according to the severity of the pain and the response of the patient. There can be considerable variability in both the dosage requirement and patient response.
When administered intravenously, meperidine hydrochloride should be given very slowly . Rapid intravenous injection increases the incidence of adverse reactions; severe respiratory depression, apnea, hypotension, peripheral circulatory collapse and cardiac arrest have occurred. This drug should be administered intravenously only if a narcotic antagonist (i.e., naloxone) and the facilities for assisted or controlled respiration are immediately available. When meperidine hydrochloride is given parenterally, especially intravenously, the patient should be lying down.
Adults: The usual initial dose for adult administration via a compatible Hospira infusion device is 10 mg, with a range of 1 to 5 mg per incremental dose. The recommended Lockout Interval is 6 to 10 minutes. The minimum recommended Lockout Interval is 5 minutes.
The physician may adjust the dosage either upward or downward; or, increase or decrease the Lockout Interval, depending on patient response. For continuous infusion the usual adult dose is 15 to 35 mg per hour administered intravenously as required.
Incompatibility: Meperidine hydrochloride is incompatible with soluble barbiturates, aminophylline, heparin, morphine sulfate, methicillin, phenytoin, sodium bicarbonate, iodide, sulfadiazine and sulfisoxazole.
Dosage of meperidine hydrochloride should be carefully adjusted according to the severity of pain and the response of the patient. Reduced dosage is indicated in poor-risk patients, in the very young or very old, in patients with impaired renal or hepatic function and in patients receiving other central nervous system depressants. For surgical patients, dosage should be based on response of the patient, other premedication and concomitant medications, the anesthetic being used and the nature and duration of the operation.
Occasionally, it may be necessary to exceed the usual dosage recommended in cases of exceptionally severe pain or in those patients who become tolerant.
Parenteral drug products should be inspected visually for particulate matter and discoloration whenever solution and container permit prior to administration.
HOW SUPPLIED
Meperidine Hydrochloride Injection, USP 10 mg/mL is supplied in a 30 mL single-dose container (List No. 6030).
This vial is only for use with a compatible Hospira PCA pump set with injector and a compatible Hospira infusion device (see directions for use supplied with the set or infuser). Store at 20 to 25ºC (68 to 77ºF). [See USP Controlled Room Temperature.]
Revised: March, 2005
©Hospira 2005 EN-0860 Printed in USA
HOSPIRA, INC., LAKE FOREST, IL 60045 USA
RL-2386
Meperidine HydrochlorideMeperidine Hydrochloride INJECTION, SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||