Mercaptopurine
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- MERCAPTOPURINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- INDICATIONS & USAGE
- MERCAPTOPURINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- MERCAPTOPURINE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
CAUTIONMercaptopurine is a potent drug. It should not be used unless a diagnosis of acute lymphatic leukemia has been adequately established and the responsible physician is experienced with the risks of mercaptopurine and knowledgeable in assessing response to chemotherapy.
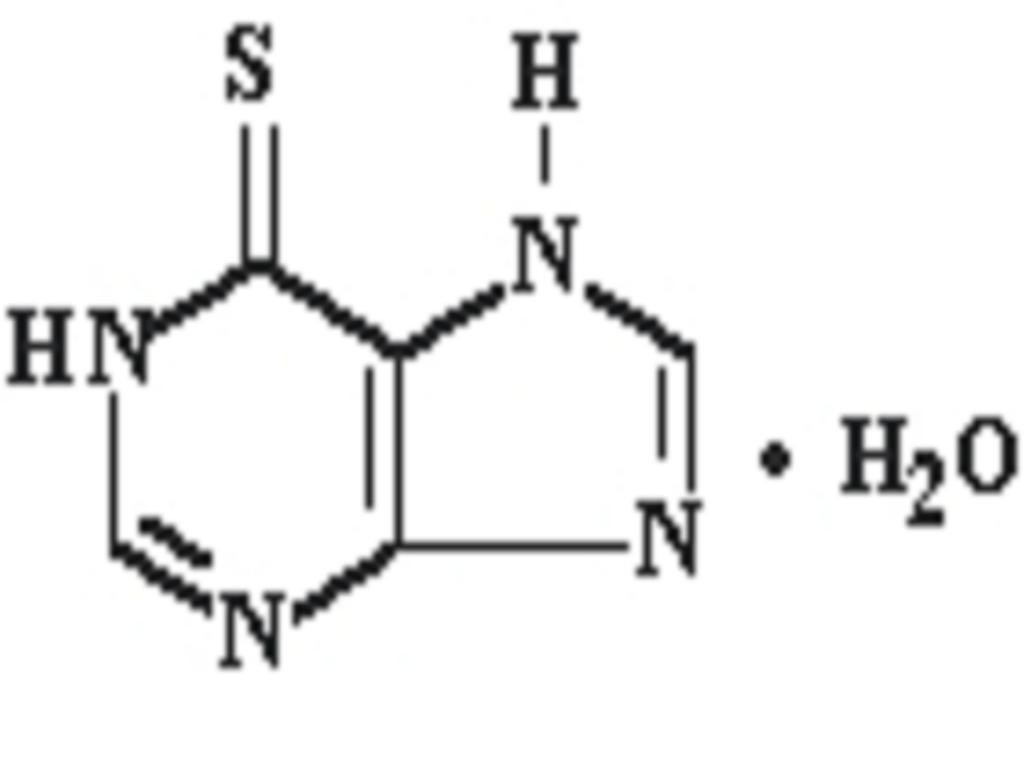
MERCAPTOPURINE DESCRIPTION
CLINICAL PHARMACOLOGY
Mechanism of actionPHARMACOKINETICS
PRECAUTIONSDOSAGE AND ADMINISTRATION
Metabolism and Genetic Polymorphism
WARNINGSPRECAUTIONSWARNINGSPRECAUTIONSWARNINGSPRECAUTIONSLaboratory TestsDOSAGE AND ADMINISTRATION
PRECAUTIONSDOSAGE AND ADMINISTRATION
INDICATIONS & USAGE
MERCAPTOPURINE CONTRAINDICATIONS
WARNINGS
Bone Marrow Toxicity
DOSAGE AND ADMINISTRATION
PRECAUTIONSDrug InteractionsDOSAGE AND ADMINISTRATION
Hepatotoxicity
Immunosuppression
Pregnancy
Pregnancy Category D
PRECAUTIONS
GeneralINFORMATION FOR PATIENTS
LABORATORY TESTS
WARNINGSBone Marrow ToxicityTPMT Testing
DRUG INTERACTIONS
WARNINGS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic EffectsPregnancy Category D
WARNINGS
NURSING MOTHERS
PEDIATRIC USE
DOSAGE AND ADMINISTRATIONGERIATRIC USE
MERCAPTOPURINE ADVERSE REACTIONS
WARNINGSPRECAUTIONSHematologic
WARNINGSBone Marrow Toxicity
Renal
Gastrointestinal
Miscellaneous
OVERDOSAGE
DOSAGE & ADMINISTRATION
Maintenance TherapyDosage with concomitant Allopurinol
Dosage in TPMT-deficient Patients
CLINICAL PHARMACOLOGYWARNINGSPRECAUTIONS
CLINICAL PHARMACOLOGYWARNINGSPRECAUTIONS
Dosage in Renal and Hepatic Impairment
HOW SUPPLIED
STORAGE AND HANDLING
Store at 20to 25(68to 77[see USP Controlled Room Temperature]. Store in a dry place. Dispense in tight container as defined in the USP.REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

MercaptopurineMercaptopurine TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!