Metfomin Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
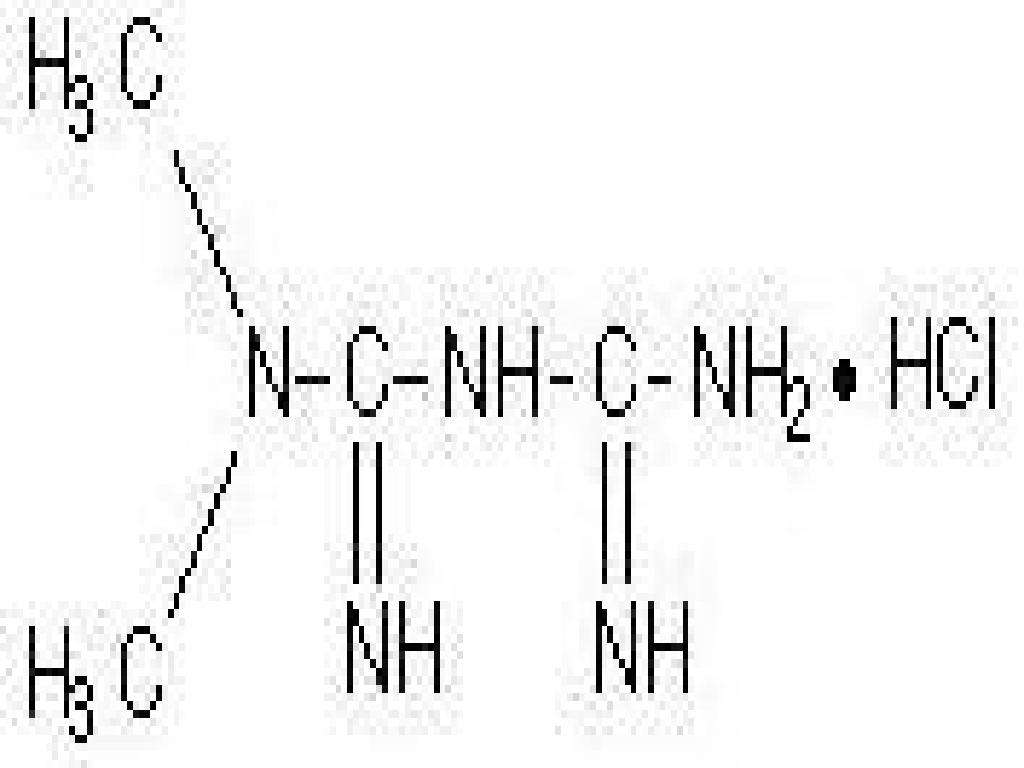
- METFOMIN HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- USE IN SPECIFIC POPULATIONS
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- METFOMIN HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- METFOMIN HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- INFORMATION FOR PATIENTS
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
BOXED WARNING
Lactic AcidosisThe reported incidence of lactic acidosis in patients receiving metformin hydrochloride is very low (approximately 0.03 cases/1000 patient-years, with approximately 0.015 fatal cases/1000 patient-years). In more than 20,000 patient-years exposure to metformin in clinical trials, there were no reports of lactic acidosis. Reported cases have occurred primarily in diabetic patients with significant renal insufficiency, including both intrinsic renal disease and renal hypoperfusion, often in the setting of multiple concomitant medical/surgical problems and multiple concomitant medications. Patients with congestive heart failure requiring pharmacologic management, in particular those with unstable or acute congestive heart failure who are at risk of hypoperfusion and hypoxemia, are at increased risk of lactic acidosis. The risk of lactic acidosis increases with the degree of renal dysfunction and the patient's age. The risk of lactic acidosis may, therefore, be significantly decreased by regular monitoring of renal function in patients taking metformin and by use of the minimum effective dose of metformin. In particular, treatment of the elderly should be accompanied by careful monitoring of renal function. Metformin treatment should not be initiated in patientsyears of age unless measurement of creatinine clearance demonstrates that renal function is not reduced, as these patients are more susceptible to developing lactic acidosis. In addition, metformin should be promptly withheld in the presence of any condition associated with hypoxemia, dehydration, or sepsis. Because impaired hepatic function may significantly limit the ability to clear lactate, metformin should generally be avoided in patients with clinical or laboratory evidence of hepatic disease. Patients should be cautioned against excessive alcohol intake, either acute or chronic, when taking metformin hydrochloride tablets, since alcohol potentiates the effects of metformin hydrochloride on lactate metabolism. In addition, metformin should be temporarily discontinued prior to any intravascular radiocontrast study and for any surgical procedure (see alsoPRECAUTIONS).
The onset of lactic acidosis often is subtle, and accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, increasing somnolence, and nonspecific abdominal distress. There may be associated hypothermia, hypotension, and resistant bradyarrhythmias with more marked acidosis. The patient and the patient's physician must be aware of the possible importance of such symptoms and the patient should be instructed to notify the physician immediately if they occur (see alsoPRECAUTIONS). Metformin hydrochloride tablets should be withdrawn until the situation is clarified. Serum electrolytes, ketones, blood glucose, and if indicated, blood pH, lactate levels, and even blood metformin levels may be useful. Once a patient is stabilized on any dose level of metformin, gastrointestinal symptoms, which are common during initiation of therapy, are unlikely to be drug related. Later occurrence of gastrointestinal symptoms could be due to lactic acidosis or other serious disease.
Levels of fasting venous plasma lactate above the upper limit of normal but less than 5 mmol/L in patients taking metformin do not necessarily indicate impending lactic acidosis and may be explainable by other mechanisms, such as poorly controlled diabetes or obesity, vigorous physical activity, or technical problems in sample handling. (See alsoPRECAUTIONS.)
Lactic acidosis should be suspected in any diabetic patient with metabolic acidosis lacking evidence of ketoacidosis (ketonuria and ketonemia).
Lactic acidosis is a medical emergency that must be treated in a hospital setting. In a patient with lactic acidosis who is taking metformin, the drug should be discontinued immediately and general supportive measures promptly instituted. Because metformin hydrochloride is dialyzable (with a clearance of up to 170 mL/min under good hemodynamic conditions), prompt hemodialysis is recommended to correct the acidosis and remove the accumulated metformin. Such management often results in prompt reversal of symptoms and recovery. (See alsoCONTRAINDICATIONSandPRECAUTIONS.)
METFOMIN HYDROCHLORIDE DESCRIPTION
CLINICAL PHARMACOLOGY
Mechanism of ActionPRECAUTIONS
PHARMACOKINETICS
Absorption and BioavailabilityDistribution
Metabolism and Elimination
Table 1
USE IN SPECIFIC POPULATIONS
Patients with Type 2 DiabetesTable 1
Renal Insufficiency
Table 1WARNINGS).
Hepatic Insufficiency
No pharmacokinetic studies of metformin have been conducted in patients with hepatic insufficiency.
Geriatrics
Limited data from controlled pharmacokinetic studies of metformin hydrochloride tablets in healthy elderly subjects suggest that total plasma clearance of metformin is decreased, the half-life is prolonged, and Cmax is increased, compared to healthy young subjects. From these data, it appears that the change in metformin pharmacokinetics with aging is primarily accounted for by a change in renal function (see Table 1). Metformin hydrochloride tablets treatment should not be initiated in patientsyears of age unless measurement of creatinine clearance demonstrates that renal function is not reduced (seeWARNINGSandDOSAGE AND ADMINISTRATION).
Table 1: Select Mean (Metformin Pharmacokinetic Parameters Following Single or Multiple Oral Doses of Metformin Hydrochloride Tablets
Subject Groups: Metformin Hydrochloride Tablets dosea (number of subjects)Cmaxb (mcg/mL)Tmaxc (hrs)Renal Clearance (mL/min)a All doses given fasting except the first 18 doses of the multiple dose studies b Peak plasma concentration c Time to peak plasma concentration d Combined results (average means) of five studies: mean age 32 years (range 23 to 59 years) e Kinetic study done following dose 19, given fasting f Elderly subjects, mean age 71 years (range 65 to 81 years) g CLcr = creatinine clearance normalized to body surface area of 1.73 m2Healthy, nondiabetic adults: 500 mg single dose (24) 850 mg single dose (74)d 850 mg three times daily for 19 dosese (9)1.03 (1.6 (2.01 (2.75 (2.64 (1.79 (600 (552 (642 (Adults with type 2 diabetes: 850 mg single dose (23) 850 mg three times daily for 19 dosese (9)1.48 (1.9 (3.32 (2.01 (491 (550 (Elderlyf, healthy nondiabetic adults: 850 mg single dose (12)2.45 (2.71 (412 (Renal-impaired adults: 850 mg single dose Mild (CLcrg 61 to 90 mL/min) (5) Moderate (CLcr 31 to 60 mL/min) (4) Severe (CLcr 10 to 30 mL/min) (6)1.86 (4.12 (3.93 (3.2 (3.75 (4.01 (384 (108 (130 (
Pediatrics
After administration of a single oral metformin hydrochloride 500 mg tablet with food, geometric mean metformin Cmax and AUC differed less than 5% between pediatric type 2 diabetic patients (12 to 16 years of age) and gender- and weight-matched healthy adults (20 to 45 years of age), all with normal renal function.
Gender
Metformin pharmacokinetic parameters did not differ significantly between normal subjects and patients with type 2 diabetes when analyzed according to gender (males = 19, females = 16). Similarly, in controlled clinical studies in patients with type 2 diabetes, the antihyperglycemic effect of metformin hydrochloride tablets was comparable in males and females.
Race
No studies of metformin pharmacokinetic parameters according to race have been performed. In controlled clinical studies of metformin hydrochloride tablets in patients with type 2 diabetes, the antihyperglycemic effect was comparable in whites (n=249), blacks (n=51), and Hispanics (n=24).
CLINICAL PHARMACOLOGY
Metformin Hydrochloride Tablets (n = 141)Placebo (n = 145)pValue
Comb (n = 213)Glyb (n = 209)MET (n =210)p-valuesGlyb vs CombMET vs CombMET vs Glyb
Metformin Hydrochloride Tablets vs PlaceboCombined Metformin Hydrochloride Tablets/Glyburide vs MonotherapyMetformin Hydrochloride Tablets (n = 141)Placebo (n = 145)Metformin Hydrochloride Tablets (n = 210)Metformin Hydrochloride Tablets/ Glyburide (n = 213)Glyburide (n = 209)
Tables 23
Metformin Hydrochloride Tablets/Insulin (n=26)Placebo/ Insulin (n=28)Treatment Difference MeanSE
Pediatric Clinical Studies
Metformin Hydrochloride TabletsPlacebop-Value
INDICATIONS & USAGE
METFOMIN HYDROCHLORIDE CONTRAINDICATIONS
WARNINGSPRECAUTIONS
PRECAUTIONS
WARNINGS
Lactic AcidosisThe reported incidence of lactic acidosis in patients receiving metformin hydrochloride is very low (approximately 0.03 cases/1000 patient-years, with approximately 0.015 fatal cases/1000 patient-years). In more than 20,000 patient-years exposure to metformin in clinical trials, there were no reports of lactic acidosis. Reported cases have occurred primarily in diabetic patients with significant renal insufficiency, including both intrinsic renal disease and renal hypoperfusion, often in the setting of multiple concomitant medical/surgical problems and multiple concomitant medications. Patients with congestive heart failure requiring pharmacologic management, in particular those with unstable or acute congestive heart failure who are at risk of hypoperfusion and hypoxemia, are at increased risk of lactic acidosis. The risk of lactic acidosis increases with the degree of renal dysfunction and the patient's age. The risk of lactic acidosis may, therefore, be significantly decreased by regular monitoring of renal function in patients taking metformin and by use of the minimum effective dose of metformin. In particular, treatment of the elderly should be accompanied by careful monitoring of renal function. Metformin treatment should not be initiated in patientsyears of age unless measurement of creatinine clearance demonstrates that renal function is not reduced, as these patients are more susceptible to developing lactic acidosis. In addition, metformin should be promptly withheld in the presence of any condition associated with hypoxemia, dehydration, or sepsis. Because impaired hepatic function may significantly limit the ability to clear lactate, metformin should generally be avoided in patients with clinical or laboratory evidence of hepatic disease. Patients should be cautioned against excessive alcohol intake, either acute or chronic, when taking metformin hydrochloride tablets, since alcohol potentiates the effects of metformin hydrochloride on lactate metabolism. In addition, metformin should be temporarily discontinued prior to any intravascular radiocontrast study and for any surgical procedure (see alsoPRECAUTIONS).
The onset of lactic acidosis often is subtle, and accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, increasing somnolence, and nonspecific abdominal distress. There may be associated hypothermia, hypotension, and resistant bradyarrhythmias with more marked acidosis. The patient and the patient's physician must be aware of the possible importance of such symptoms and the patient should be instructed to notify the physician immediately if they occur (see alsoPRECAUTIONS). Metformin hydrochloride tablets should be withdrawn until the situation is clarified. Serum electrolytes, ketones, blood glucose, and if indicated, blood pH, lactate levels, and even blood metformin levels may be useful. Once a patient is stabilized on any dose level of metformin, gastrointestinal symptoms, which are common during initiation of therapy, are unlikely to be drug related. Later occurrence of gastrointestinal symptoms could be due to lactic acidosis or other serious disease.
Levels of fasting venous plasma lactate above the upper limit of normal but less than 5 mmol/L in patients taking metformin do not necessarily indicate impending lactic acidosis and may be explainable by other mechanisms, such as poorly controlled diabetes or obesity, vigorous physical activity, or technical problems in sample handling. (See alsoPRECAUTIONS.)
Lactic acidosis should be suspected in any diabetic patient with metabolic acidosis lacking evidence of ketoacidosis (ketonuria and ketonemia).
Lactic acidosis is a medical emergency that must be treated in a hospital setting. In a patient with lactic acidosis who is taking metformin, the drug should be discontinued immediately and general supportive measures promptly instituted. Because metformin hydrochloride is dialyzable (with a clearance of up to 170 mL/min under good hemodynamic conditions), prompt hemodialysis is recommended to correct the acidosis and remove the accumulated metformin. Such management often results in prompt reversal of symptoms and recovery. (See alsoCONTRAINDICATIONSandPRECAUTIONS.)
PRECAUTIONS
GeneralWARNINGSDOSAGE AND ADMINISTRATION
PRECAUTIONS: Drug Interactions
CONTRAINDICATIONS
PRECAUTIONS: Laboratory Tests
WARNINGS
INFORMATION FOR PATIENTS
WARNINGSPRECAUTIONS
Patient Information
LABORATORY TESTS
DOSAGE AND ADMINISTRATIONDRUG INTERACTIONS
(Clinical Evaluation of Drug Interactions Conducted With Metformin Hydrochloride Tablets)DOSAGE AND ADMINISTRATION: Concomitant Metformin and Oral Sulfonylurea Therapy in Adult Patients
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic effectsNURSING MOTHERS
PEDIATRIC USE
CLINICAL PHARMACOLOGY: Pediatric Clinical StudiesADVERSE REACTIONS: Pediatric PatientsDOSAGE AND ADMINISTRATION: Recommended Dosing Schedule: PediatricsGERIATRIC USE
CONTRAINDICATIONSWARNINGSCLINICAL PHARMACOLOGY: PharmacokineticsWARNINGSDOSAGE AND ADMINISTRATIONMETFOMIN HYDROCHLORIDE ADVERSE REACTIONS
Adverse ReactionMetformin Hydrochloride Tablets Monotherapy (n = 141)Placebo (n = 145)% of Patients
Pediatric Patients
OVERDOSAGE
WARNINGSDOSAGE & ADMINISTRATION
Recommended Dosing Schedule
Recommended Dosing Schedule
Transfer From Other Antidiabetic Therapy
Concomitant Metformin and Oral Sulfonylurea Therapy in Adult Patients
CLINICAL PHARMACOLOGY: Clinical Studies
Concomitant Metformin and Insulin Therapy in Adult Patients
Specific Patient Populations
WARNINGS
HOW SUPPLIED
STORAGE AND HANDLING
INFORMATION FOR PATIENTS
Metformin Hydrochloride Tablets, USPWhat is metformin?
What are the side effects of metformin?
Who should not take metformin?
-
● have kidney problems
-
● have liver problems
-
● have heart failure that is treated with medicines, such as Lanoxin(digoxin) or Lasix(furosemide)
-
● drink a lot of alcohol. This means you binge drink for short periods or drink all the time
-
● are seriously dehydrated (have lost a lot of water from your body)
-
● are going to have an x-ray procedure with injection of dyes (contrast agents)
-
● are going to have surgery
-
● develop a serious condition, such as heart attack, severe infection, or a stroke
-
● are 80 years or older and you have NOT had your kidney function tested
Can metformin hydrochloride tablets be used in children?
How should I take metformin hydrochloride tablets?
-
● have an illness that causes severe vomiting, diarrhea or fever, or if you drink a much lower amount of liquid than normal. These conditions can lead to severe dehydration (loss of water in your body). You may need to stop taking metformin for a short time.
-
● plan to have surgery or an x-ray procedure with injection of dye (contrast agent). You may need to stop taking metformin hydrochloride tablets for a short time.
-
● start to take other medicines or change how you take a medicine. Metformin can affect how well other drugs work, and some drugs can affect how well metformin work. Some medicines may cause high blood sugar.
What should I avoid while taking metformin hydrochloride tablets?
What are the side effects of metformin?
-
● feeling very weak, tired, or uncomfortable
-
● unusual muscle pain
-
● trouble breathing
-
● unusual or unexpected stomach discomfort
-
● feeling cold
-
● feeling dizzy or lightheaded
-
● suddenly developing a slow or irregular heartbeat
General advice about prescription medicines
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Metfomin HydrochlorideMetformin Hydrochloride TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!