Metformin Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
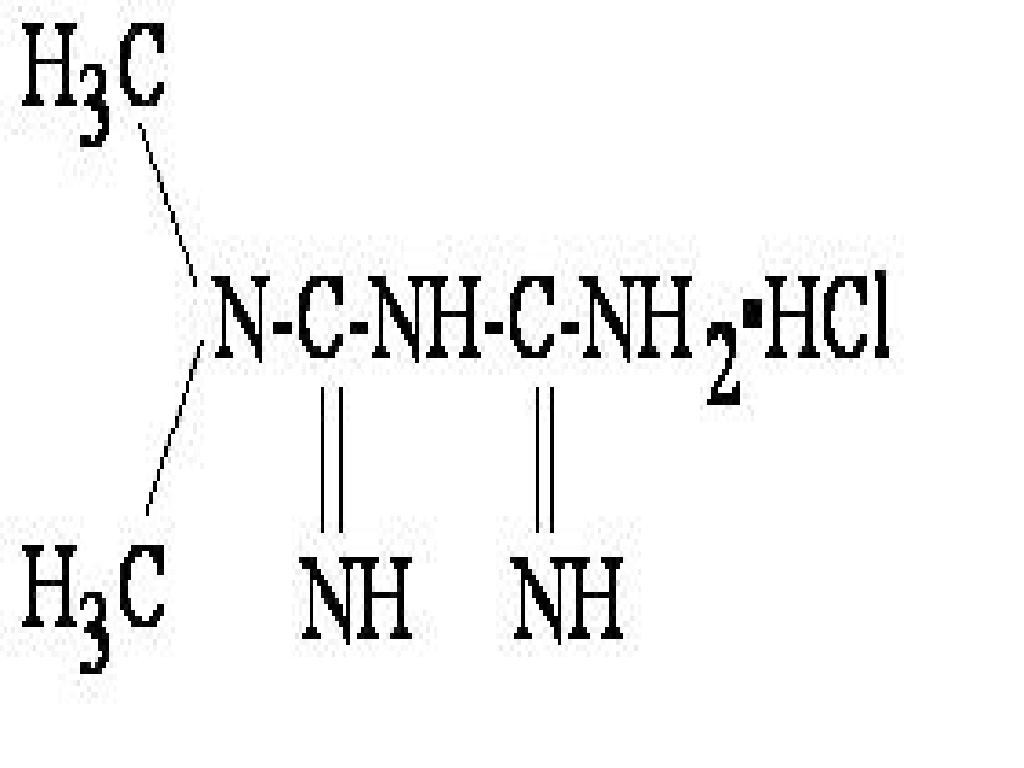
- METFORMIN HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- USE IN SPECIFIC POPULATIONS
- CLINICAL STUDIES
- INDICATIONS & USAGE
- METFORMIN HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- METFORMIN HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- INFORMATION FOR PATIENTS
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
Lactic Acidosis:Lactic acidosis is a rare, but serious, metabolic complication that can occur due to metformin accumulation during treatment with metformin; when it occurs, it is fatal in approximately 50% of cases. Lactic acidosis may also occur in association with a number of pathophysiologic conditions, including diabetes mellitus, and whenever there is significant tissue hypoperfusion and hypoxemia. Lactic acidosis is characterized by elevated blood lactate levels (>5 mmol/L), decreased blood pH, electrolyte disturbances with an increased anion gap, and an increased lactate/pyruvate ratio. When metformin is implicated as the cause of lactic acidosis, metformin plasma levels >5are generally found.
The reported incidence of lactic acidosis in patients receiving metformin hydrochloride is very low (approximately 0.03 cases/1000 patient-years, with approximately 0.015 fatal cases/ 1000 patient-years). In more than 20,000 patient-years exposure to metformin in clinical trials, there were no reports of lactic acidosis. Reported cases have occurred primarily in diabetic patients with significant renal insufficiency, including both intrinsic renal disease and renal hypoperfusion, often in the setting of multiple concomitant medical/surgical problems and multiple concomitant medications. Patients with congestive heart failure requiring pharmacologic management, in particular those with unstable or acute congestive heart failure who are at risk of hypoperfusion and hypoxemia, are at increased risk of lactic acidosis. The risk of lactic acidosis increases with the degree of renal dysfunction and the patient's age. The risk of lactic acidosis may, therefore, be significantly decreased by regular monitoring of renal function in patients taking metformin and by use of the minimum effective dose of metformin. In particular, treatment of the elderly should be accompanied by careful monitoring of renal function. Metformin treatment should not be initiated in patientsyears of age unless measurement of creatinine clearance demonstrates that renal function is not reduced, as these patients are more susceptible to developing lactic acidosis. In addition, metformin should be promptly withheld in the presence of any condition associated with hypoxemia, dehydration, or sepsis. Because impaired hepatic function may significantly limit the ability to clear lactate, metformin should generally be avoided in patients with clinical or laboratory evidence of hepatic disease. Patients should be cautioned against excessive alcohol intake, either acute or chronic, when taking metformin, since alcohol potentiates the effects of metformin hydrochloride on lactate metabolism. In addition, metformin should be temporarily discontinued prior to any intravascular radiocontrast study and for any surgical procedure (see alsoPRECAUTIONS).
The onset of lactic acidosis often is subtle, and accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, increasing somnolence, and nonspecific abdominal distress. There may be associated hypothermia, hypotension, and resistant bradyarrhythmias with more marked acidosis. The patient and the patient's physician must be aware of the possible importance of such symptoms and the patient should be instructed to notify the physician immediately if they occur (see alsoPRECAUTIONS). Metformin should be withdrawn until the situation is clarified. Serum electrolytes, ketones, blood glucose, and if indicated, blood pH, lactate levels, and even blood metformin levels may be useful. Once a patient is stabilized on any dose level of metformin, gastrointestinal symptoms, which are common during initiation of therapy, are unlikely to be drug related. Later occurrence of gastrointestinal symptoms could be due to lactic acidosis or other serious disease.
Levels of fasting venous plasma lactate above the upper limit of normal but less than 5 mmol/L in patients taking metformin do not necessarily indicate impending lactic acidosis and may be explainable by other mechanisms, such as poorly controlled diabetes or obesity, vigorous physical activity, or technical problems in sample handling (see alsoPRECAUTIONS).
Lactic acidosis should be suspected in any diabetic patient with metabolic acidosis lacking evidence of ketoacidosis (ketonuria and ketonemia).
Lactic acidosis is a medical emergency that must be treated in a hospital setting. In a patient with lactic acidosis who is taking metformin, the drug should be discontinued immediately and general supportive measures promptly instituted. Because metformin hydrochloride is dialyzable (with a clearance of up to 170 mL/min under good hemodynamic conditions), prompt hemodialysis is recommended to correct the acidosis and remove the accumulated metformin. Such management often results in prompt reversal of symptoms and recovery (see alsoCONTRAINDICATIONSandPRECAUTIONS).
METFORMIN HYDROCHLORIDE DESCRIPTION
System Components and Performance:
CLINICAL PHARMACOLOGY
Mechanism of ActionPRECAUTIONS
PHARMACOKINETICS
Absorption and BioavailabilityDistribution
Metabolism and Elimination
USE IN SPECIFIC POPULATIONS
Renal Insufficiency
WARNINGS
Hepatic Insufficiency
Geriatrics
WARNINGSDOSAGE AND ADMINISTRATION
Pediatrics
Gender
Race
CLINICAL STUDIES
Metformin hydrochloride tabletsMetformin hydrochloride extended-release tablets
DOSAGE AND ADMINISTRATION
*
*DOSAGE AND ADMINISTRATION
*
*
*
*
Pediatric Clinical Studies
INDICATIONS & USAGE
METFORMIN HYDROCHLORIDE CONTRAINDICATIONS
WARNINGSPRECAUTIONS
PRECAUTIONS
WARNINGS
Lactic Acidosis:Lactic acidosis is a rare, but serious, metabolic complication that can occur due to metformin accumulation during treatment with metformin; when it occurs, it is fatal in approximately 50% of cases. Lactic acidosis may also occur in association with a number of pathophysiologic conditions, including diabetes mellitus, and whenever there is significant tissue hypoperfusion and hypoxemia. Lactic acidosis is characterized by elevated blood lactate levels (>5 mmol/L), decreased blood pH, electrolyte disturbances with an increased anion gap, and an increased lactate/pyruvate ratio. When metformin is implicated as the cause of lactic acidosis, metformin plasma levels >5are generally found.
The reported incidence of lactic acidosis in patients receiving metformin hydrochloride is very low (approximately 0.03 cases/1000 patient-years, with approximately 0.015 fatal cases/ 1000 patient-years). In more than 20,000 patient-years exposure to metformin in clinical trials, there were no reports of lactic acidosis. Reported cases have occurred primarily in diabetic patients with significant renal insufficiency, including both intrinsic renal disease and renal hypoperfusion, often in the setting of multiple concomitant medical/surgical problems and multiple concomitant medications. Patients with congestive heart failure requiring pharmacologic management, in particular those with unstable or acute congestive heart failure who are at risk of hypoperfusion and hypoxemia, are at increased risk of lactic acidosis. The risk of lactic acidosis increases with the degree of renal dysfunction and the patient's age. The risk of lactic acidosis may, therefore, be significantly decreased by regular monitoring of renal function in patients taking metformin and by use of the minimum effective dose of metformin. In particular, treatment of the elderly should be accompanied by careful monitoring of renal function. Metformin treatment should not be initiated in patientsyears of age unless measurement of creatinine clearance demonstrates that renal function is not reduced, as these patients are more susceptible to developing lactic acidosis. In addition, metformin should be promptly withheld in the presence of any condition associated with hypoxemia, dehydration, or sepsis. Because impaired hepatic function may significantly limit the ability to clear lactate, metformin should generally be avoided in patients with clinical or laboratory evidence of hepatic disease. Patients should be cautioned against excessive alcohol intake, either acute or chronic, when taking metformin, since alcohol potentiates the effects of metformin hydrochloride on lactate metabolism. In addition, metformin should be temporarily discontinued prior to any intravascular radiocontrast study and for any surgical procedure (see alsoPRECAUTIONS).
The onset of lactic acidosis often is subtle, and accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, increasing somnolence, and nonspecific abdominal distress. There may be associated hypothermia, hypotension, and resistant bradyarrhythmias with more marked acidosis. The patient and the patient's physician must be aware of the possible importance of such symptoms and the patient should be instructed to notify the physician immediately if they occur (see alsoPRECAUTIONS). Metformin should be withdrawn until the situation is clarified. Serum electrolytes, ketones, blood glucose, and if indicated, blood pH, lactate levels, and even blood metformin levels may be useful. Once a patient is stabilized on any dose level of metformin, gastrointestinal symptoms, which are common during initiation of therapy, are unlikely to be drug related. Later occurrence of gastrointestinal symptoms could be due to lactic acidosis or other serious disease.
Levels of fasting venous plasma lactate above the upper limit of normal but less than 5 mmol/L in patients taking metformin do not necessarily indicate impending lactic acidosis and may be explainable by other mechanisms, such as poorly controlled diabetes or obesity, vigorous physical activity, or technical problems in sample handling (see alsoPRECAUTIONS).
Lactic acidosis should be suspected in any diabetic patient with metabolic acidosis lacking evidence of ketoacidosis (ketonuria and ketonemia).
Lactic acidosis is a medical emergency that must be treated in a hospital setting. In a patient with lactic acidosis who is taking metformin, the drug should be discontinued immediately and general supportive measures promptly instituted. Because metformin hydrochloride is dialyzable (with a clearance of up to 170 mL/min under good hemodynamic conditions), prompt hemodialysis is recommended to correct the acidosis and remove the accumulated metformin. Such management often results in prompt reversal of symptoms and recovery (see alsoCONTRAINDICATIONSandPRECAUTIONS).
PRECAUTIONS
GeneralWARNINGSDOSAGE AND ADMINISTRATION
PRECAUTIONS
CONTRAINDICATIONS
PRECAUTIONS
WARNINGS
INFORMATION FOR PATIENTS
WARNINGSPRECAUTIONS
LABORATORY TESTS
DOSAGE AND ADMINISTRATIONDRUG INTERACTIONS
DOSAGE AND ADMINISTRATION
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic Effects: Pregnancy Category BNURSING MOTHERS
PEDIATRIC USE
CLINICAL PHARMACOLOGYADVERSE REACTIONSDOSAGE AND ADMINISTRATIONGERIATRIC USE
CONTRAINDICATIONSWARNINGSCLINICAL PHARMACOLOGYWARNINGSDOSAGE AND ADMINISTRATIONMETFORMIN HYDROCHLORIDE ADVERSE REACTIONS
*
*
*
*
Pediatric Patients
OVERDOSAGE
WARNINGSDOSAGE & ADMINISTRATION
Recommended Dosing Schedule
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY
Specific Patient Populations
WARNINGS
HOW SUPPLIED
STORAGE AND HANDLING
INFORMATION FOR PATIENTS
What are metformin hydrochloride tablets and metformin hydrochloride extended-release tablets?
Who should not take metformin hydrochloride tablets and metformin hydrochloride extended-release tablets?
-
● have kidney problems
-
● have liver problems
-
● have heart failure that is treated with medicines, such as Lanoxin(digoxin) or Lasix(furosemide)
-
● drink a lot of alcohol. This means you binge drink for short periods or drink all the time
-
● are seriously dehydrated (have lost a lot of water from your body)
-
● are going to have an x-ray procedure with injection of dyes (contrast agents)
-
● are going to have surgery
-
● develop a serious condition, such as heart attack, severe infection, or a stroke
-
● are 80 years or older and you have NOT had your kidney function tested
Can metformin hydrochloride tablets or metformin hydrochloride extended-release tablets be used in children?
How should I take metformin hydrochloride tablets or metformin hydrochloride extended-release tablets?
-
● have an illness that causes severe vomiting, diarrhea or fever, or if you drink a much lower amount of liquid than normal. These conditions can lead to severe dehydration (loss of water in your body). You may need to stop taking metformin hydrochloride tablets or metformin hydrochloride extended-release tablets for a short time.
-
● plan to have surgery or an x-ray procedure with injection of dye (contrast agent). You may need to stop taking metformin hydrochloride tablets or metformin hydrochloride extended-release tablets for a short time.
-
● start to take other medicines or change how you take a medicine. Metformin hydrochloride tablets or metformin hydrochloride extended-release tablets can affect how well other drugs work, and some drugs can affect how well metformin hydrochloride tablets or metformin hydrochloride extended-release tablets work. Some medicines may cause high blood sugar.
What should I avoid while taking metformin hydrochloride tablets or metformin hydrochloride extended-release tablets?
What are the side effects of metformin hydrochloride tablets and metformin hydrochloride extended-release tablets?
-
● feeling very weak, tired, or uncomfortable
-
● unusual muscle pain
-
● trouble breathing
-
● unusual or unexpected stomach discomfort
-
● feeling cold
-
● feeling dizzy or lightheaded
-
● suddenly developing a slow or irregular heartbeat
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Metformin HydrochlorideMetformin Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!