Metformin Hydrochloride Extended Release
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- METFORMIN HYDROCHLORIDE EXTENDED RELEASE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- METFORMIN HYDROCHLORIDE EXTENDED RELEASE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- METFORMIN HYDROCHLORIDE EXTENDED RELEASE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- SPL PATIENT PACKAGE INSERT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
METFORMIN HYDROCHLORIDE EXTENDED RELEASE DESCRIPTION
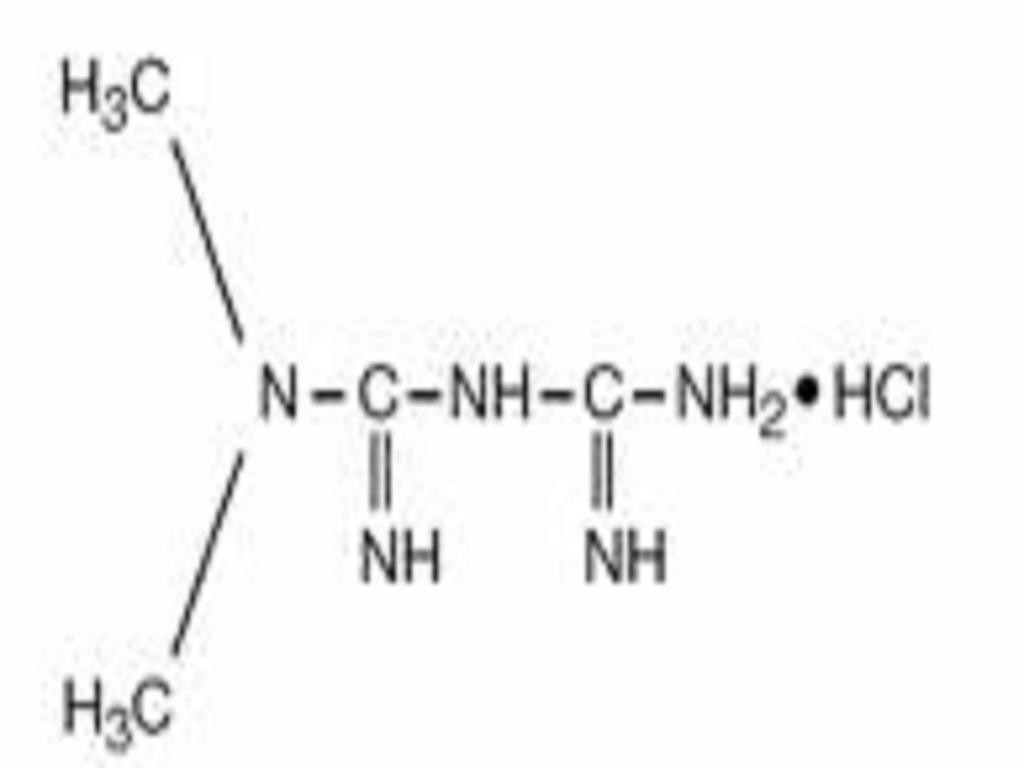
CLINICAL PHARMACOLOGY
Mechanism of ActionPharmacokinetics
Absorption and Bioavailability
Distribution
Metabolism and Elimination
Special Populations
Patients with Type 2 Diabetes
Renal Insufficiency
WARNINGS
Hepatic Insufficiency
Geriatrics
Subject Groups: MetforminCmaxbTmaxcRenal ClearanceHydrochloride tablets dose a(ug/mL)(hrs)(mL/min)(number of subjects)Healthy, nondiabetic adults:+++++++++Adults with type 2 diabetes:++++++Elderlyf, healthy nondiabetic adults:Renal-impaired adults:850 mg single doseMild+++Moderate+++Severe+++
Pediatrics
Gender
Race
Clinical Studies
Metformin Hydrochloride Tablet
Metformin HClPlacebopValue(n=141)(n=145)FPG (mg/dL)Hemoglobin A1c (%)Body Weight (lbs)
p-valuesCombGlybGLUGlyb vsGLU vsGLU vs(n=213)(n=209)(n=210)CombCombGlybFast PlasmaGlucose (mg/dL)Hemoglobin A1c (%)Body Weight (lbs)
Metformin vs PlaceboCombined Metformin/Glyburidevs MonotherapyMetformin/MetforminPlaceboMetforminGlyburideGlyburide(n=141)(n=145)(n=210)(n=213)(n=209)Total Cholesterol (mg/dL)Total Triglycerides (mg/dL)LDL-Cholesterol (mg/dL)HDL-Cholesterol (mg/dL)
Metformin/ InsulinPlacebo/ InsulinTreatment Difference(n=26)(n=28)Mean+SEHemoglobin A1c (%)Baseline8.959.32Change at FINAL VISIT-2.10-1.56-0.54+0.43aInsulin Dose (U/day)+
Metformin Hydrochloride Extended-Release Tablets
Metformin Hydrochloride ExtendedRelease Tablets500 mg1000 mg1500 mg2000 mg1000 mgPlaceboOnce DailyOnce DailyOnce DailyOnce DailyTwice DailyHemoglobin A1c (%)(n=115)(n=115)(n=111)(n=125)(n=112)(n=111)FPG (mg/dL)(n=126)(n=118)(n=120)(n=132)(n=122)(n=113)Body Weight (lbs)(n=125)(n=119)(n=117)(n=131)(n=119)(n=113)
Metformin HydrochlorideMetformin HydroclorideTabletsExtended Release Tablets500 mg Twice Daily1000 mg Once Daily1500 mg Once DailyHemoglobin A1c(%) (n=67)(n=72)(n=66)FPG (mg/dL)(n=69)(n=72)(n=70)Body Weight (lbs)(n=71)(n=74)(n=71)
Metformin Hydrochloride Extended-Release Tablets500 mg1000 mg1500 mg2000 mg1000mgPlaceboOnceOnceOnceOnceTwiceDailyDailyDailyDailyDailyTotal Cholesterol (mg/dL)(n=120)(n=113)(n=110)(n=126)(n=117)(n=110)Total Triglycerides (mg/dL)(n=120)(n=113)(n=110)(n=126)(n=117)(n=110)LDL-Cholesterol (mg/dL)(n=119)(n=113)(n=109)(n=126)(n=117)(n=107)HDL-Cholesterol (mg/dL)(n=120)(n=108)(n=108)(n=125)(n=117)(n=108)
GLUCOPHAGEMetformin HydrochlorideExtended - Release Tablets500 mg1000 mg1500 mgTwice DailyOnce DailyOnce DailyTotal Cholesterol (mg/dL)(n=68)(n=70)(n=66)Total Triglycerides(mg/dL)(n=68)(n=70)(n=66)LDL-Cholesterol (mg/dL)(n=68)(n=70)(n=66)HDL-Cholesterol (mg/dL)(n=68)(n=70)(n=65)
Pediatric Clinical Studies
MetforminPlacebop-ValueFPG (mg/dL)(n=37)(n=36)Body Weight (lbs)(n=39)(n=38)
INDICATIONS & USAGE
METFORMIN HYDROCHLORIDE EXTENDED RELEASE CONTRAINDICATIONS
-
● Renal disease or renal dysfunction (e.g., as suggested by serum creatinine levelsmg/dL [males],mg/dL [females] or abnormal creatinine clearance) which may also result from conditions such as cardiovascular collapse (shock), acute myocardial infarction, and septicemia (seeWARNINGSandPRECAUTIONS).
-
● Known hypersensitivity to metformin hydrochloride.
-
● Acute or chronic metabolic acidosis, including diabetic ketoacidosis, with or without coma. Diabetic ketoacidosis should be treated with insulin.
-
● Metformin hydrochloride and Metformin hydrochloride extended-release tablets should be temporarily discontinued in patients undergoing radiologic studies involving intravascular administration of iodinated contrast materials, because use of such products may result in acute alteration of renal function (see also PRECAUTIONS).
WARNINGS
PRECAUTIONS
GeneralPRECAUTIONS: Drug Interactions
INFORMATION FOR PATIENTS
LABORATORY TESTS
DRUG INTERACTIONS
(clinical evaluation of drug interactions done with metformin hydrochloride tablets)CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic Effects: Pregnancy Category BNURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
METFORMIN HYDROCHLORIDE EXTENDED RELEASE ADVERSE REACTIONS
Adverse ReactionMetformin HCl MonotherapyPlacebo(n=141)(n=145)% of Patients
Metformin Hydrochloride ExtendedPlaceboRelease Tablets (n=781)(n=195)Adverse Reaction% of Patients
Pediatric Patients
OVERDOSAGE
WARNINGSDOSAGE & ADMINISTRATION
Recommended Dosing Schedule
Transfer From Other Antidiabetic Therapy
Concomitant Metformin and Oral Sulfonylurea Therapy In Adult Patients
Concomitant Metformin and Insulin Therapy in Adult Patients
Specific Patient Populations
STORAGE AND HANDLING
SPL PATIENT PACKAGE INSERT
BOXED WARNING SECTIONPACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Metformin Hydrochloride Extended ReleaseMetformin Hydrochloride Extended Release TABLET, EXTENDED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!