Methadone Hydrochloride
Methadone Hydrochloride Tablets, USP CII10 mgRx OnlyAscend Laboratories
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- METHADONE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- METHADONE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- General
- Drug Interactions
- Opioid Antagonists, Mixed Agonist/Antagonists, and Partial Agonists
- Anti-retroviral Agents
- Cytochrome P450 Inducers
- Cytochrome P450 Inhibitors
- Others
- Potentially Arrhythmogenic Agents
- Interactions with Alcohol and Drugs of Abuse
- Risk of Relapse in Patients on Methadone Maintenance Treatment of Opioid Addiction
- Tolerance and Physical Dependence
- Special-Risk Patients
- Information for Patients
- Carcinogenesis & Mutagenesis & Impairment Of Fertility
- Pregnancy
- Labor & Delivery
- Nursing Mothers
- Pediatric Use
- Geriatric Use
- Renal Impairment
- METHADONE HYDROCHLORIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PATIENT INFORMATION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
BOXED WARNING
Deaths, cardiac and respiratory, have been reported during initiation and conversion of pain patients to methadone treatment from treatment with other opioid agonists. It is critical to understand the pharmacokinetics of methadone when converting patients from other opioids (see DOSAGE AND ADMINISTRATION). Particular vigilance is necessary during treatment initiation, during conversion from one opioid to another, and during dose titration.
Respiratory depression is the chief hazard associated with methadone hydrochloride administration. Methadone's peak respiratory depressant effects typically occur later, and persist longer than its peak analgesic effects, particularly in the early dosing period. These characteristics can contribute to cases of iatrogenic overdose, particularly during treatment initiation and dose titration.
In addition, cases of QT interval prolongation and serious arrhythmia (torsades de pointes) have been observed during treatment with methadone. Most cases involve patients being treated for pain with large, multiple daily doses of methadone, although cases have been reported in patients receiving doses commonly used for maintenance treatment of opioid addiction.
Methadone treatment for analgesic therapy in patients with acute or chronic pain should only be initiated if the potential analgesic or palliative care benefit of treatment with methadone is considered and outweighs the risks.
Conditions For Distribution And Use Of Methadone Products For The Treatment Of Opioid Addiction
- During inpatient care, when the patient was admitted for any condition other than concurrent opioid addiction (pursuant to 21CFR 1306.07(c)), to facilitate the treatment of the primary admitting diagnosis).
- During an emergency period of no longer than 3 days while definitive care for the addiction is being sought in an appropriately licensed facility (pursuant to 21CFR 1306.07(b)).
METHADONE HYDROCHLORIDE DESCRIPTION
2127
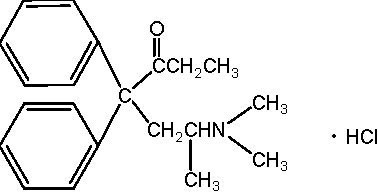
Other ingredients of methadone include: magnesium stearate, microcrystalline cellulose, and starch.
CLINICAL PHARMACOLOGY
Mechanism of Action
Some data also indicate that methadone acts as an antagonist at the N-methyl-D-aspartate (NMDA) receptor. The contribution of NMDA receptor antagonism to methadone’s efficacy is unknown. Other NMDA receptor antagonists have been shown to produce neurotoxic effects in animals.
Pharmacokinetics
Absorption
Distribution
Metabolism
Excretion
1/2
Pharmacokinetics in Special Populations
Pregnancy
PRECAUTIONS: Pregnancy, Labor and Delivery, DOSAGE AND ADMINISTRATION
Renal Impairment
Hepatic Impairment
Gender
Race
Geriatric
Pediatric
Drug Interactions PRECAUTIONS, Drug Interactions
INDICATIONS & USAGE
- For the treatment of moderate to severe pain not responsive to non-narcotic analgesics.
- For detoxification treatment of opioid addiction (heroin or other morphine-like drugs).
- For maintenance treatment of opioid addiction (heroin or other morphine-like drugs), in conjunction with appropriate social and medical services.
NOTE
Outpatient maintenance and outpatient detoxification treatment may be provided only by Opioid Treatment Programs (OTPs) certified by the Federal Substance Abuse and Mental Health Services Administration (SAMHSA) and registered by the Drug Enforcement Administration (DEA). This does not preclude the maintenance treatment of a patient with concurrent opioid addiction who is hospitalized for conditions other than opioid addiction and who requires temporary maintenance during the critical period of his/her stay, or of a patient whose enrollment has been verified in a program which has been certified for maintenance treatment with methadone.
METHADONE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
Respiratory Depression, Incomplete Cross-tolerance, and Iatrogenic Overdose Respiratory depression is the chief hazard associated with methadone hydrochloride administration. Methadone's peak respiratory depressant effects typically occur later, and persist longer than its peak analgesic effects, particularly during the initial dosing period. These characteristics can contribute to cases of iatrogenic overdose, particularly during treatment initiation or dose titration.
Patients tolerant to other opioids may be incompletely tolerant to methadone. Incomplete cross-tolerance is of particular concern for patients tolerant to other mu-opioid agonists who are being converted to treatment with methadone, thus making determination of dosing during opioid treatment conversion complex. Deaths have been reported during conversion from chronic, high-dose treatment with other opioid agonists. Therefore, it is critical to understand the pharmacokinetics of methadone when converting patients from other opioids (see DOSAGE AND ADMINISTRATION, Tables 1 and 2, for appropriate conversion schedules). A high degree of "opioid tolerance" does not eliminate the possibility of methadone overdose, iatrogenic or otherwise.
Cardiac Conduction Effects
in vivoin vitro
Misuse, Abuse, and Diversion of Opioids
Interactions with other CNS Depressants
PRECAUTIONS
Interactions with Alcohol and Drugs of Abuse
Head Injury and Increased Intracranial Pressure
Acute Abdominal Conditions
Hypotensive Effect
DRUG ABUSE AND DEPENDENCE
Methadone is a mu-agonist opioid with an abuse liability similar to other opioid agonists and is a Schedule II controlled substance. Methadone and other opioids used in analgesia can be abused and are subject to criminal diversion.
Physical Dependence and Tolerance
PRECAUTIONS; Pregnancy, Labor and Delivery
PRECAUTIONS
General
INDICATIONS AND USAGEDOSAGE AND ADMINISTRATION
Drug Interactions
In vitro results suggest that methadone undergoes hepatic N-demethylation by cytochrome P450 enzymes, principally CYP3A4, CYP2B6, CYP2C19 and to a lesser extent by CYP2C9 and CYP2D6. Coadministration of methadone with CYP inducers of these enzymes may result in a more rapid metabolism and potential for decreased effects of methadone, whereas administration with CYP inhibitors may reduce metabolism and potentiate methadone’s effects. Although antiretroviral drugs such as efavirenz, nelfinavir, nevirapine, ritonavir, lopinavir+ritonavir combination are known to inhibit CYPs, they are shown to reduce the plasma levels of methadone, possibly due to their CYP induction activity. Therefore, drugs administered concomitantly with methadone should be evaluated for interaction potential; clinicians are advised to evaluate individual response to drug therapy.
Opioid Antagonists, Mixed Agonist/Antagonists, and Partial Agonists
As with other mu-agonists, patients maintained on methadone may experience withdrawal symptoms when given opioid antagonists, mixed agonist/antagonists, and partial agonists. Examples of such agents are naloxone, naltrexone, pentazocine, nalbuphine, butorphanol, and buprenorphine.
Anti-retroviral Agents
Cytochrome P450 Inducers
Rifampin
Phenytoin
St. John’s Wort, Phenobarbital, Carbamazepine
Cytochrome P450 Inhibitors
Voriconazole – Repeat dose administration of oral voriconazole (400mg Q12h for 1 day, then 200mg Q12h for 4 days) increased the Cmax and AUC of (R)-methadone by 31% and 47%, respectively, in subjects receiving a methadone maintenance dose (30 to 100 mg QD). The Cmax and AUC of (S)-methadone increased by 65% and 103%, respectively. Increased plasma concentrations of methadone have been associated with toxicity including QT prolongation. Frequent monitoring for adverse events and toxicity related to methadone is recommended during coadministration. Dose reduction of methadone may be needed.
Others
Monoamine Oxidase (MAO) Inhibitors
Desipramine – Blood levels of desipramine have increased with concurrent methadone administration.
Potentially Arrhythmogenic Agents
Caution should also be exercised when prescribing methadone concomitantly with drugs capable of inducing electrolyte disturbances (hypomagnesemia, hypokalemia) that may prolong the QT interval. These drugs include diuretics, laxatives, and, in rare cases, mineralocorticoid hormones.
Interactions with Alcohol and Drugs of Abuse
Anxiety
Acute Pain – Maintenance patients on a stable dose of methadone who experience physical trauma, postoperative pain or other acute pain cannot be expected to derive analgesia from their existing dose of methadone. Such patients should be administered analgesics, including opioids, in doses that would otherwise be indicated for non-methadone-treated patients with similar painful conditions. Due to the opioid tolerance induced by methadone, when opioids are required for management of acute pain in methadone patients, somewhat higher and/or more frequent doses will often be required than would be the case for non-tolerant patients.
Risk of Relapse in Patients on Methadone Maintenance Treatment of Opioid Addiction
Abrupt opioid discontinuation can lead to development of opioid withdrawal symptoms (see PRECAUTIONS). Presentation of these symptoms have been associated with an increased risk of susceptible patients to relapse to illicit drug use and should be considered when assessing the risks and benefit of methadone use.
Tolerance and Physical Dependence
In general, chronically administered methadone should not be abruptly discontinued.
Special-Risk Patients
Methadone should be given with caution and the initial dose reduced in certain patients, such as the elderly and debilitated and those with severe impairment of hepatic or renal function, hypothyroidism, Addison’s disease, prostatic hypertrophy, or urethral stricture. The usual precautions appropriate to the use of parenteral opioids should be observed and the possibility of respiratory depression should always be kept in mind.
Information for Patients
- Patients should be cautioned that methadone, like all opioids, may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving or operating machinery.
- Patients should be cautioned that methadone, like other opioids, may produce orthostatic hypotension in ambulatory patients.
- Patients should be cautioned that alcohol and other CNS depressants may produce an additive CNS depression when taken with this product and should be avoided.
- Patients should be instructed to seek medical attention immediately if they experience symptoms suggestive of an arrhythmia (such as palpitations, dizziness, lightheadedness, or syncope) when taking methadone.
- Patients initiating treatment with methadone for opioid dependence should be reassured that the dose of methadone will “hold” for longer periods of time as treatment progresses.
- Patients seeking to discontinue methadone maintenance treatment of opioid dependence should be apprised of the high risk of relapse to illicit drug use associated with discontinuation of methadone maintenance treatment.
- Patients should be instructed to keep methadone in a secure place out of the reach of children and other household members. Accidental or deliberate ingestion by a child may cause respiratory depression that can result in death. Patients and their caregivers should be advised to discard unused methadone in such a way that individuals other than the patient for whom it was originally prescribed will not come in contact with the drug.
Carcinogenesis & Mutagenesis & Impairment Of Fertility
2
in vivoin vivoE. coliNeurospora crassa
Pregnancy
Teratogenic EffectsPregnancy Category C
222
Nonteratogenetic Effects
CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATION
Methadone should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Labor & Delivery
As with all opioids, administration of this product to the mother shortly before delivery may result in some degree of respiratory depression in the newborn, especially if higher doses are used. Methadone is not recommended for obstetric analgesia because its long duration of action increases the probability of respiratory depression in the newborn. Narcotics with mixed agonist-antagonist properties should not be used for pain control during labor in patients chronically treated with methadone as they may precipitate acute withdrawal.
Nursing Mothers
Because of the potential for serious adverse reactions in nursing infants from methadone, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. In patients being treated for opioid dependence, this should include weighing the risk of methadone against the risk of maternal illicit drug use.
Pediatric Use
Accidental or deliberate ingestion by a child may cause respiratory depression that can result in death. Patients and caregivers should be instructed to keep methadone in a secure place out of the reach of children and to discard unused methadone in such a way that individuals other than the patient for whom it was originally prescribed will not come in contact with the drug.
Geriatric Use
Clinical studies of methadone did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently compared to younger subjects. Other reported clinical experience has not identified differences in responses between elderly and younger patients. In general, dose selection for elderly patients should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
Renal Impairment
The use of methadone has not been extensively evaluated in patients with renal insufficiency.
Hepatic Impairment
The use of methadone has not been extensively evaluated in patients with hepatic insufficiency. Methadone is metabolized in the liver and patients with liver impairment may be at risk of accumulating methadone after multiple dosing.
Gender
The use of methadone has not been evaluated for gender specificity.
METHADONE HYDROCHLORIDE ADVERSE REACTIONS
Heroin Withdrawal
Initial Administration
The major hazards of methadone are respiratory depression and, to a lesser degree, systemic hypotension. Respiratory arrest, shock, cardiac arrest, and death have occurred.
most frequently observed adverse reactions
Body as a Whole – asthenia (weakness), edema, headache
DRUG ABUSE AND DEPENDENCE
Methadone contains methadone, a mu-agonist opioid with an abuse liability similar to other opioid agonists and is a Schedule II controlled substance. Methadone and other opioids used in analgesia have the potential for being abused and are subject to criminal diversion.
ABUSE
“Drug-seeking” behavior is very common in addicts and drug abusers. Drug-seeking tactics include emergency calls or visits near the end of office hours, refusal to undergo appropriate examination, testing or referral, repeated claims of lost prescriptions, tampering with prescriptions and reluctance to provide prior medical records or contact information for other treating physician(s). “Doctor shopping” (visiting multiple prescribers) to obtain additional prescriptions is common among drug abusers and people suffering from untreated addiction. However, it should be important to note that preoccupation with achieving adequate pain relief can be appropriate behavior in a patient with poor pain control.
PHYSICAL DEPENDENCE AND TOLERANCE
PRECAUTIONS; Pregnancy, Labor and Delivery
Treatment
The physician must remember, however, that methadone is a long-acting depressant (36 to 48 hours), whereas opioid antagonists act for much shorter periods (one to three hours)
Oxygen, intravenous fluids, vasopressors, and other supportive measures should be employed as indicated.
DOSAGE & ADMINISTRATION
Particular vigilance is necessary during treatment initiation, during conversion from one opioid to another, and during dose titration
Methadone's peak respiratory depressant effects typically occur later, and persist longer than its peak analgesic effects
The complexities associated with methadone dosing can contribute to cases of iatrogenic overdose, particularly during treatment initiation and dose titration. A high degree of "opioid tolerance" does not eliminate the possibility of methadone overdose, iatrogenic or otherwise. Deaths have been reported during conversion to methadone from chronic, high-dose treatment with other opioid agonists and during initiation of methadone treatment of addiction in subjects previously abusing high doses of other agonists.
Treatment of Pain
imprecise
- The total daily dose, potency and specific characteristics of the opioid the patient had been taking previously, if any;
- The relative potency estimate used to calculate an equianalgesic starting methadone dose, in particular, whether it is intended for use in acute or chronic methadone dosing;
- The patient’s degree of opioid tolerance;
- The age, general condition and medical status of the patient;
- Concurrent medications, particularly other CNS and respiratory depressants;
- The type, severity and expected duration of the patient’s pain;
- The acceptable balance between pain control and adverse side effects.
Initiation of Therapy in Opioid Non-Tolerant Patients
When oral methadone is used as the first analgesic in patients who are not already being treated with, and tolerant to, opioids, the usual oral methadone starting dose is 5 mg to 10 mg every 8 to 12 hours, slowly titrated to effect. More frequent administration may be required during methadone initiation in order to maintain adequate analgesia, and extreme caution is necessary to avoid overdosage, taking into account methadone’s long elimination half-life.
Conversion from Parenteral Methadone to Oral Methadone
Conversion from parenteral methadone to oral methadone should initially use a 1:2 dose ratio (e.g., 5 mg parenteral methadone to 10 mg oral methadone).
Switching Patients to Methadone from other Chronic Opioids
Deaths have occurred in opioid tolerant patients during conversion to methadone
| Total Daily Baseline
Oral
Morphine Dose |
Estimated Daily
Oral
Methadone Requirement as Percent of Total Daily Morphine Dose |
| <100 mg | 20% to 30% |
| 100 to 300 mg | 10% to 20% |
| 300 to 600 mg | 8% to 12% |
| 600 mg to 1000 mg | 5% to 10% |
| >1000 mg | <5% |
Note: Equianalgesic methadone dosing varies not only between patients, but also within the same patient, depending on baseline morphine (or other opioid) dose. Table 1 has been included in order to illustrate this concept and to provide a safe starting point for opioid conversion. Methadone dosing should not be based solely on these tables. Methadone conversion and dose titration methods should always be individualized to account for the patient’s prior opioid exposure, general medical condition, concomitant medication, and anticipated breakthrough medication use. The endpoint of titration is achievement of adequate pain relief, balanced against tolerability of opioid side effects. If a patient develops intolerable opioid related side effects, the methadone dose, or dosing interval, may need to be adjusted.
Dosage Adjustment During Pregnancy
Methadone clearance may be increased during pregnancy. Several small studies have demonstrated significantly lower trough methadone plasma concentrations and shorter methadone half-lives in women during their pregnancy compared to after their delivery. During pregnancy a woman’s methadone dose may need to be increased, or their dosing interval decreased. Methadone should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus.
Detoxification and Maintenance Treatment of Opiate Dependence
| For detoxification and maintenance of opiate dependence methadone should be administered in accordance with the treatment standards cited in 42 CFR Section 8.12, including limitations on unsupervised administration |
For Short-term Detoxification
For Maintenance Treatment
For Medically Supervised Withdrawal After a Period of Maintenance Treatment
There is considerable variability in the appropriate rate of methadone taper in patients choosing medically supervised withdrawal from methadone treatment. It is generally suggested that dose reductions should be less than 10% of the established tolerance or maintenance dose, and that 10 to 14-day intervals should elapse between dose reductions. Patients should be apprised of the high risk of relapse to illicit drug use associated with discontinuation of methadone maintenance treatment.
HOW SUPPLIED
DEA order form required
PATIENT INFORMATION
Methadone Hydrochloride Tablets, USP CII
WARNINGS: Keep methadone hydrochloride tablets out of the reach of children. Accidental overdose by a child is a medical emergency and can result in death. If a child accidentally takes methadone hydrochloride tablets, get emergency help right away. Do not take a higher dose of methadone hydrochloride tablets or take it more often than prescribed. This can lead to an overdose and possible death.
What Is The Most Important Information I Should Know About methadone hydrochloride tablets?
- Methadone hydrochloride tablets can cause life threatening breathing problems which can lead to death. These problems are more likely to happen when methadone hydrochloride tablets is first started or in someone who is not already taking other narcotic (opioid) pain medicines.
- Breathing problems from methadone hydrochloride tablets may not happen right away after taking a dose. Sometimes breathing problems will happen a while after you take a dose, even after pain has returned. It is very important that you take methadone hydrochloride tablets exactly as your doctor has prescribed. Talk to your doctor about your pain. Your doctor can decide if your methadone hydrochloride tablets dose needs to be changed.
- Methadone hydrochloride tablets can cause life-threatening heart beat problems that can lead to death.
What is methadone hydrochloride tablets?
Methadone hydrochloride tablets is a federally controlled substance (CII) because it is a strong opioid pain medicine that can be abused by people who abuse prescriptionmedicines or street drugs
Prevent theft and misuse. Keep your methadone hydrochloride tablets in a safe placeSelling or giving away this medicineis dangerous and against the law. Methadone hydrochloride tablets is used:
- to treat moderate to severe pain in people that do not respond to non-narcotic pain medicines;
- to control withdrawal symptoms in patients being treated for narcotic drug addiction;
- for maintenance treatment of narcotic drug addiction along with other social and medical services. Stopping maintenance treatment of narcotic drug addiction with methadone hydrochloride tablets may result in a return to narcotic drug use.
Who Should Not Take methadone hydrochloride tablets? Do not take methadone hydrochloride tablets if you:
- have severe asthma or severe lung problems.
- have a blockage or obstruction in your intestines.
- are allergic to methadone or anything else in methadone hydrochloride tablets. See the end of this leaflet for a complete list of ingredients.
What Should I Tell my Doctor Before I Start Taking Methadone Hydrochloride Tablets?
Methadone hydrochloride tablets may not be right for you. Before starting methadone hydrochloride tablets, tell your doctor about all your medical and mental conditions including a history of drug or alcohol abuse or addiction. Tell your doctor if you:
- are pregnant or plan to become pregnant. Methadone hydrochloride tablets may harm your unborn baby.
- are breast feeding. Methadone hydrochloride tablets passes through your breast milk and may harm your baby. You should choose to use methadone hydrochloride tablets or breastfeed, but not both. Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Some medicines may cause serious or lifethreatening medical problems when taken with Methadone hydrochloride tablets. Be especially careful about other medicines that may make you sleepy, such as other pain medicines, antidepressantmedicines, sleeping pills, anxiety medicines, antihistamines, or tranquilizers. Sometimes, the doses of certain medicines (including Methadone hydrochloride tablets) may need to be changed if they are used together.
Do not take any medicine while using methadone hydrochloride tablets until you have first talked to your doctor or pharmacist. They will be able to tell you if it is safe to take other medicines while you are using methadone hydrochloride tablets
How Should I Take Methadone Hydrochloride Tablets?
- Take methadone hydrochloride tablets exactly as prescribed. Follow your doctor's directions exactly. Your doctor may change your dose based on your reactions to the medicine. Do not change your dose unless your doctor tells you to change it. Do not take a higher dose of methadone hydrochloride tablets or take it more often than prescribed. This can lead to an overdose and possibly death.
- If you take too much methadone hydrochloride tablets or overdose, call 911 or your local emergency number right away.
- Review your medical conditions regularly with your doctor to determine if you still need methadone hydrochloride tablets, or if the dose needs to be adjusted.
- When starting treatment with methadone hydrochloride tablets for narcotic drug dependence, you should be aware that your dose of methadone will “hold” for longer periods of time as treatment goes on.
- Stopping Methadone Hydrochloride Tablets. Ask your doctor for instructions on how to stop this medicine slowly to avoid uncomfortable symptoms. You should not stop taking methadone hydrochloride tablets all at once if you have been taking it for more than a few days.
- Tell all health professionals that treat you that you take methadone hydrochloride tablets.
- After stopping treatment with methadone hydrochloride tablets, flush the unused tablets down the toilet.
What Should I Avoid While Taking Methadone Hydrochloride Tablets?
- Do not drive, operate heavy machinery, or do other possible dangerous activities until you know how methadone hydrochloride tablets affects you. Methadone hydrochloride tablets can make you sleepy. Ask your doctor when it is okay to do these activities.
- Do not drink alcohol while using methadone hydrochloride tablets. It may increase the chance of having dangerous side effects.
- Do not take other medicines with methadone hydrochloride tablets without first talking with your doctor. What are the Possible Side Effects of Methadone Hydrochloride Tablets?
- Methadone hydrochloride tablets can cause life threatening breathing and heart problems which can lead to death
- Call your doctor or get medical help right away if you:
- have trouble breathing
- have extreme drowsiness and breathing slows down
- have slow shallow breathing (little chest movement with breathing)
- fast or slowed heartbeat
- feel faint, very dizzy, confused, have palpitations (irregular heart beat) or any other unusual symptoms These can be symptoms that you have taken too much (overdose of) methadone hydrochloride tablets, or the dose is too high for you. They can also be symptoms of a serious heart reaction.
These symptoms can lead to serious problems or death if not treated right away.
- Methadone hydrochloride tablets can cause your blood pressure to drop. This can make you feel dizzy if you get up too fast from sitting or lying down.
- Methadone hydrochloride tablets can cause physical dependence. Do not stop taking methadone hydrochloride tablets or any other opioid without first talking to your doctor. You could become sick with uncomfortable withdrawal symptoms because your body has become used to these medicines. Talk to your doctor about slowly stopping methadone hydrochloride tablets to avoid getting sick with withdrawal symptoms. Physical dependency is not the same as drug addiction.
- For patients using methadone hydrochloride tablets for pain treatment, there is a chance of abuse or addiction with methadone hydrochloride tablets. The chance is higher if you are or have been addicted to or abused other medicines, street drugs, or alcohol, or if you have a history of mental problems. Some common side effects of methadone hydrochloride tablets are lightheadedness, dizziness, drowsiness, nausea, vomiting and sweating. Other side effects include weakness, headache, constipation, itching, and dry mouth. Talk to your doctor about any side effects that bother you or that do not go away. These are not all the possible side effects of methadone hydrochloride tablets. For a complete list, ask your doctor or pharmacist.
How Should I Store Methadone Hydrochloride Tablets?
- Keep Methadone hydrochloride tablets in a safe place away from children. Accidental use by a child is a medical emergency that can result in death. If a child accidentally takes methadone hydrochloride tablets, get emergency help right away.
- Keep methadone hydrochloride tablets at room temperature, 20°-25°C (68°-77°F)[See USP Controlled Room Temperature].
- Always keep methadone hydrochloride tablets in a secure place to protect from theft.
- Dispose of any unused methadone hydrochloride tablets remaining from a prescription as soon as they are no longer needed. Unused tablets should be flushed down the toilet.
General Information About Methadone Hydrochloride Tablets.
Do not give methadone hydrochloride tablets to other people, even if they have the same symptoms you have. Methadone hydrochloride tablets can harm other people and even cause death. Sharing methadone hydrochloride tablets is against the law
What are the Ingredients in methadone hydrochloride tablets? Active Ingredient:Inactive Ingredients:
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
ASCEND LABORATORIES, LLC

Methadone HydrochlorideMethadone Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||