Metoclopramide Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- METOCLOPRAMIDE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- INDICATIONS & USAGE
- METOCLOPRAMIDE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- METOCLOPRAMIDE HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- SPL MEDGUIDE
- INACTIVE INGREDIENT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
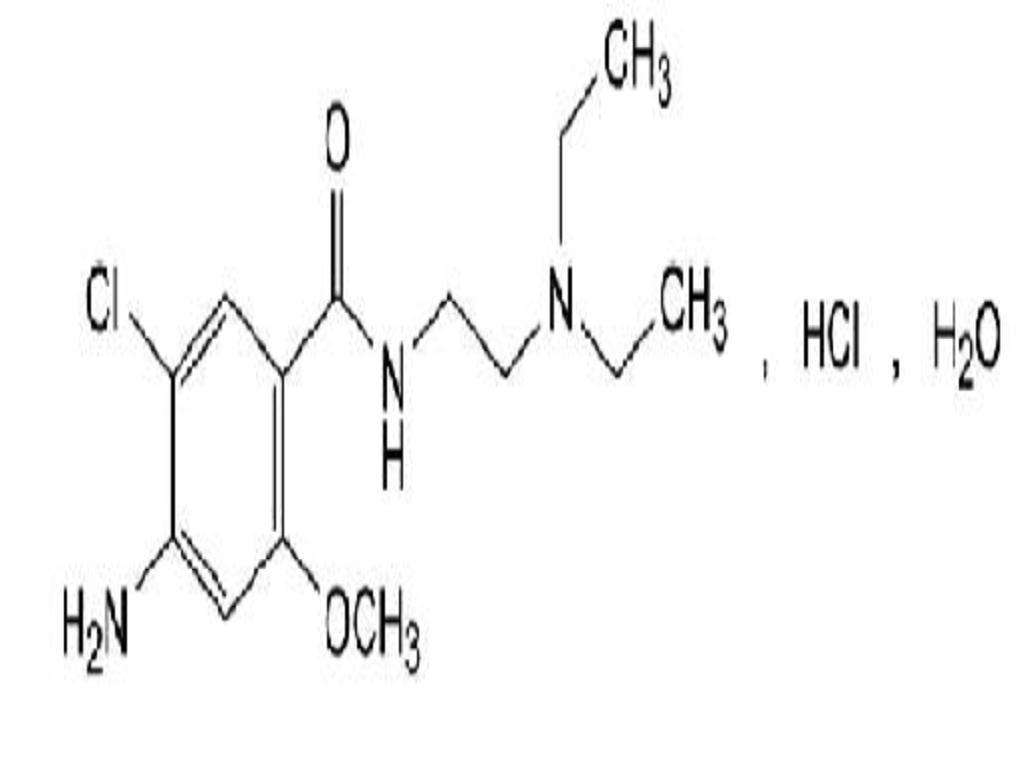
METOCLOPRAMIDE HYDROCHLORIDE DESCRIPTION
DESCRIPTIONInactive Ingredients
Inactive Ingredients
CLINICAL PHARMACOLOGY
WARNINGS
PHARMACOKINETICS
Adult Pharmacokinetic Data
ParameterValue
INDICATIONS & USAGE
Symptomatic Gastroesophageal Reflux
Diabetic Gastroparesis (Diabetic Gastric Stasis)
METOCLOPRAMIDE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
Tardive Dyskinesia
(see Boxed Warnings)
Neuroleptic Malignant Syndrome(NMS)
ADVERSE REACTIONS
PRECAUTIONS
GeneralINFORMATION FOR PATIENTS
DRUG INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Pregnancy Category BNURSING MOTHERS
PEDIATRIC USE
OVERDOSAGECLINICAL PHARMACOLOGYPharmacokineticsOVERDOSAGE
WARNINGSADVERSE REACTIONSExtrapyramidal Reactions
GERIATRIC USE
WARNINGSDOSAGE AND ADMINISTRATIONFor the Relief of Symptomatic Gastroesophageal Reflux
WARNINGSTardive Dyskinesia
CLINICAL PHARMACOLOGYPRECAUTIONS-Information for PatientsADVERSE REACTIONSCNS Effects
DOSAGE AND ADMINISTRATION - Use in Patients with Renal or Hepatic Impairment
DOSAGE AND ADMINISTRATIONFor the Relief of Symptomatic Gastroesophageal RefluxUse in Patients with Renal or Hepatic Impairment
Other Special Populations
OVERDOSAGE
METOCLOPRAMIDE HYDROCHLORIDE ADVERSE REACTIONS
CNS Effects
PRECAUTIONSWARNINGS
Extrapyramidal Reactions (EPS)
WARNINGS
WARNINGSWARNINGS
Neuroleptic Malignant Syndrome
WARNINGS
Endocrine Disturbances
PRECAUTIONSCLINICAL PHARMACOLOGY
Cardiovascular
CONTRAINDICATIONSPRECAUTIONS
Gastrointestinal
Hepatic
Renal
Hematologic
OVERDOSAGE
Allergic Reactions
Miscellaneous
OVERDOSAGE
PRECAUTIONSOther Special Populations
DOSAGE & ADMINISTRATION
For the Relief of Symptomatic Gastroesophageal Reflux
CLINICAL PHARMACOLOGYINDICATIONS AND USAGE
ADVERSE REACTIONS
For the Relief of Symptoms Associated with Diabetic Gastroparesis (Diabetic Gastric Stasis)
Use in Patients with Renal or Hepatic Impairment
OVERDOSAGE
HOW SUPPLIED
STORAGE AND HANDLING
SPL MEDGUIDE
MEDICATION GUIDE-
● the longer you take metoclopramide tablets and the more metoclopramide tablets you take. You should not take metoclopramide tablets for more than 12 weeks.
-
● if you are older, especially if you are a woman
-
● if you have diabetes
-
● It is not possible for your doctor to know if you will get TD if you take metoclopramide tablets.
-
● frowning or scowling
-
● sticking out your tongue
-
● blinking and moving your eyes
-
● shaking of your arms and legs
-
● See the section "What are the possible side effects of metoclopramide tablets?" for more information about side effects.
-
● to relieve symptoms of slow stomach emptying in people with diabetes. Metoclopramide tablets help treat symptoms such as nausea, vomiting, heartburn, feeling full long after a meal, and loss of appetite. Not all these symptoms get better at the same time.
-
● It is not known if metoclopramide tablets are safe and work in children.
-
● have an adrenal gland tumor called a pheochromocytoma
-
● are allergic to metoclopramide tablets or anything in it. See the end of this Medication Guide for a list of ingredients in metoclopramide tablets.
-
● take medicines that can cause uncontrolled movements, such as medicines for mental illness
-
● have seizures
-
● What should I tell my doctor before taking metoclopramide tablets?
-
● Parkinson's disease
-
● high blood pressure
-
● kidney problems. Your doctor may start with a lower dose.
-
● liver problems or heart failure. Metoclopramide tablets may cause your body to hold fluids.
-
● diabetes. Your dose of insulin may need to be changed.
-
● breast cancer
-
● you are pregnant or plan to become pregnant. It is not known if metoclopramide tablets will harm your unborn baby.
-
● you are breast-feeding. Metoclopramide tablets can pass into breast milk and may harm your baby. Talk with your doctor about the best way to feed your baby if you take metoclopramide tablets.
-
● Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Metoclopramide tablets and some other medicines may interact with each other and may not work as well, or cause possible side effects. Do not start any new medicines while taking metoclopramide tablets until you talk with your doctor.
-
● a blood pressure medicine
-
● a medicine for depression, especially an Monoamine Oxidase Inhibitor (MAOI)
-
● insulin
-
● a medicine that can make you sleepy, such an anti-anxiety medicine, sleep medicines, and narcotics.
-
● If you are not sure if your medicine is one listed above, ask your doctor or pharmacist.
-
● Take metoclopramide tablets exactly as your doctor tells you. Do not change your dose unless your doctor tells you.
-
● You should not take metoclopramide tablets for more than 12 weeks.
-
● If you take too much metoclopramide tablets, call your doctor or Poison Control Center right away.
-
● What should I avoid while taking metoclopramide tablets?
-
● Do not drive, work with machines, or do dangerous tasks until you know how metoclopramide tablets affect you. Metoclopramide tablets may cause sleepiness.
-
● What are the possible side effects of metoclopramide tablets?
-
● Uncontrolled spasms of your face and neck muscles, or muscles of your body, arms, and legs (dystonia). These muscle spasms can cause abnormal movements and body positions. These spasms usually start within the first 2 days of treatment. These spasms happen more often in children and adults under age 30.
-
● Depression, thoughts about suicide, and suicide. Some people who take metoclopramide tablets become depressed. You may have thoughts about hurting or killing yourself. Some people who take metoclopramide tablets have ended their own lives (suicide).
-
● Neuroleptic Malignant Syndrome (NMS). NMS is a very rare but very serious condition that can happen with metoclopramide tablets. NMS can cause death and must be treated in a hospital. Symptoms of NMS include: high fever, stiff muscles, problems thinking, very fast or uneven heartbeat, and increased sweating.
-
● Parkinsonism. Symptoms include slight shaking, body stiffness, trouble moving or keeping your balance. If you already have Parkinson's disease, your symptoms may become worse while you are receiving metoclopramide tablets.
-
● Call your doctor and get medical help right away if you:
-
● have high fever, stiff muscles, problems thinking, very fast or uneven heartbeat, and increased sweating
-
● have muscle movements you cannot stop or control
-
● have muscle movements that are new or unusual
-
● Common side effects of metoclopramide tablets include:
-
● headache
-
● confusion
-
● trouble sleeping
-
● You may have more side effects the longer you take metoclopramide tablets and the more metoclopramide tablets you take.
-
● Keep metoclopramide tablets in the bottle it comes in. Keep the bottle closed tightly.
-
● Keep metoclopramide tablets and all medicines out of the reach of children.
INACTIVE INGREDIENT
INACTIVE INGREDIENTS:CELLULOSE, MICROCRYSTALLINE
COLLOIDAL SILICON DIOXIDE
STARCH, CORN
STARCH, CORN
STEARIC ACID
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Metoclopramide HydrochlorideMetoclopramide Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!