METOPROLOL SUCCINATE
These highlights do not include all the information needed to use metoprolol succinate extended-release tablets safely and effectively. See full prescribing information for metoprolol succinate extended-release tablets. Metoprolol Succinate Extended-Release Tablets, USP for Oral use. INITIAL U.S. APPROVAL:1992
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- DOSAGE FORMS & STRENGTHS
- METOPROLOL SUCCINATE CONTRAINDICATIONS
- WARNINGS AND PRECAUTIONS
- METOPROLOL SUCCINATE ADVERSE REACTIONS
- DRUG INTERACTIONS
- USE IN SPECIFIC POPULATIONS
- OVERDOSAGE
- METOPROLOL SUCCINATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- NONCLINICAL TOXICOLOGY
- CLINICAL STUDIES
- HOW SUPPLIED
- INFORMATION FOR PATIENTS
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
WARNING: ISCHEMIC HEART DISEASE:
Following abrupt cessation of therapy with certain beta-blocking agents, exacerbations of angina pectoris and, in some cases, myocardial infarction have occurred. When discontinuing chronically administered metoprolol succinate extended-release tablets, particularly in patients with ischemic heart disease, the dosage should be gradually reduced over a period of 1 - 2 weeks and the patient should be carefully monitored. If angina markedly worsens or acute coronary insufficiency develops, metoprolol succinate extended-release tablets administration should be reinstated promptly, at least temporarily, and other measures appropriate for the management of unstable angina should be taken. Warn patients against interruption or discontinuation of therapy without the physician’s advice. Because coronary artery disease is common and may be unrecognized, it may be prudent not to discontinue metoprolol succinate extended-release tablets therapy abruptly even in patients treated only for hypertension (5.1).
INDICATIONS & USAGE
[see Dosage and Administration (2)].
DOSAGE & ADMINISTRATION
Adults:
Pediatric Hypertensive Patients ≥ 6 Years of age:[see Use in Specific Populations (8.4)][see Clinical Pharmacology (12.3)]
[see Use in Specific Populations (8.4)].
[see Warnings and Precautions (5)].
DOSAGE FORMS & STRENGTHS
METOPROLOL SUCCINATE CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
[see Dosage and Administration (2)].
112[see Dosage and Administration (2)].
METOPROLOL SUCCINATE ADVERSE REACTIONS
- Worsening angina or myocardial infarction. [see Warnings and Precautions (5)]
- Worsening heart failure. [see Warnings and Precautions (5)]
- Worsening AV block. [see Contraindications (4)]
Heart Failure:
Adverse Reactions Occurring in the MERIT-HF Study at an Incidence ≥ 1 % in the Metoprolol Succinate Extended-Release Tablets Group and Greater Than Placebo by More Than 0.5 %
|
|
Metoprolol succinate extended-release tablets n=1990% of patients |
Placebo n=2001% of patients |
|---|---|---|
| Dizziness/vertigo |
1.8 |
1.0 |
| Bradycardia |
1.5 |
0.4 |
| Accident and/or injury |
1.4 |
0.8 |
Cardiovascular:
Respiratory:
Central Nervous System:
Gastrointestinal:
Hypersensitive Reactions:
Miscellaneous:
Potential Adverse Reactions:
Central Nervous System:
Hematologic:
Hypersensitive Reactions:
DRUG INTERACTIONS
[see WARNINGS AND PRECAUTIONS (5.11)].
USE IN SPECIFIC POPULATIONS
2
- Dose-response for reduction in DBP,
- 1.0 mg/kg vs. placebo for change in SBP, and
- 2.0 mg/kg vs. placebo for change in SBP and DBP.
[see Clinical Pharmacology (12.3)].
OVERDOSAGE
2
METOPROLOL SUCCINATE DESCRIPTION
1
CLINICAL PHARMACOLOGY
Hypertension:
Heart Failure:
12
1211
max1112
2
Adults:
[see Drug Interactions (7.2)].
1[see Clinical Pharmacology (12)].
Pediatrics:
NONCLINICAL TOXICOLOGY
22
SalmonellaSalmonella
2
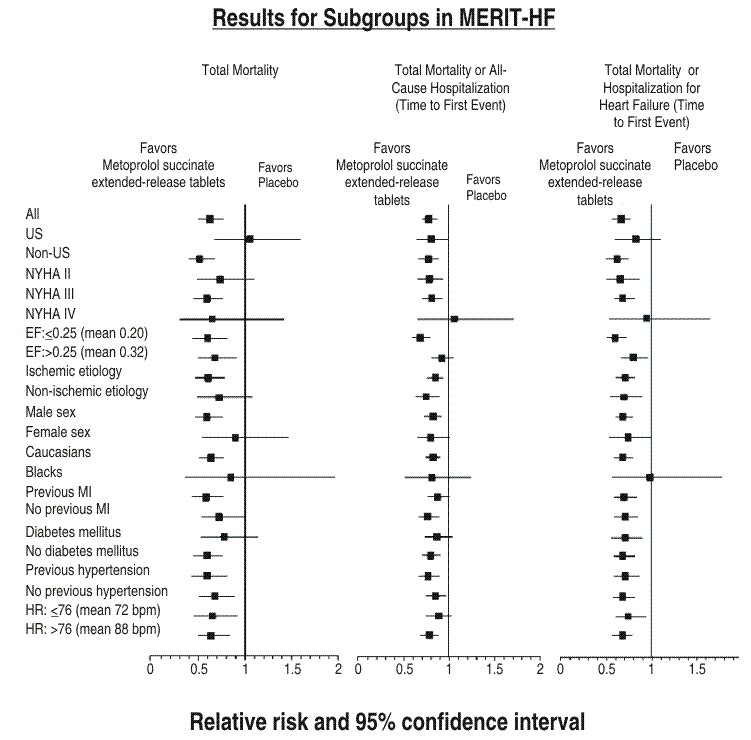
CLINICAL STUDIES
11111111®
1
|
Clinical Endpoint |
Number of Patients |
Relative Risk (95% Cl) |
Risk Reduction With Metoprolol Succinate Extended-Release Tablets |
Nominal P-value |
|
|
Placebo n=2001 |
Metoprolol Succinate Extended-Release Tablets n=1990 |
||||
|
All-cause mortality plus all-caused hospitalization* |
767 |
641 |
0.81(0.73- 0.90) |
19% |
0.00012 |
|
All-cause mortality |
217 |
145 |
0.66(0.53- 0.81) |
34% |
0.00009 |
|
All-cause mortality plus heart failure hospitalization |
439 |
311 |
0.69(0.60- 0.80) |
31% |
0.0000008 |
|
Cardiovascular mortality |
203 |
128 |
0.62(0.50- 0.78) |
38% |
0.000022 |
|
Sudden death |
132 |
79 |
0.59(0.45- 0.78) |
41% |
0.0002 |
|
Death due to worsening heart failure |
58 |
30 |
0.51(0.33- 0.79) |
49% |
0.0023 |
|
Hospitalizations due to worsening heart failure† |
451 |
317 |
N/A |
N/A |
0.0000076 |
|
Cardiovascular hospitalization |
773 |
649 |
N/A |
N/A |
0.00028 |
|
|


HOW SUPPLIED
| Tablet |
Shape |
Debossed |
Bottle of 30 NDC 64679- |
Bottle of 100 NDC 64679- |
Bottle of 500 NDC 64679- |
Unit Dose Packages of 100 NDC 64679- |
| 25 mg* |
Oval |
W and 34 |
734-01 |
734-02 |
734-03 | 734-05 |
| 50 mg |
Circular, beveled edge |
W 735 |
735-01 |
735-02 |
735-03 | 735-05 |
| 100 mg |
Circular, beveled edge | W 736 |
736-01 |
736-02 |
736-03 | 736-05 |
| 200 mg |
Oval, beveled edge | W737 |
737-01 |
737-02 |
737-03 | 737-05 |
INFORMATION FOR PATIENTS
Manufactured by:
Distributed by:
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION


METOPROLOL SUCCINATEMETOPROLOL SUCCINATE TABLET, FILM COATED, EXTENDED RELEASE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||