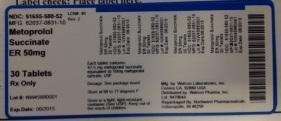
Metoprolol Succinate
Northwind Pharmaceuticals
Northwind Pharmaceuticals
FULL PRESCRIBING INFORMATION
NDC: 51655-580-52
MFG: 62037-0831-10
Metoprolol Succinate ER 50 MG
30 Tablets
Rx Only
Lot# NW45880001
Exp: Date: 06/2015
Each tablet contains: 47.5 mg metoprolol succinate equivalent
to 50mg metoprolol tartate, USP
Dosage: See package insert
Store at 68 to 77 degrees F.
Store in a tight, light-resistant container (See USP).
Keep out of the reach of children.
Mfg by: Watson Laboratories, Inc Corona, CA 92880 USA
Distributed by: Watson Pharma, Incc Lot: 847064A
Repackaged by: Northwind Pharmaceuticals, Indianapolis, IN 46256
Metoprolol SuccinateMetoprolol Succinate TABLET, FILM COATED, EXTENDED RELEASE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!