Metoprolol Tartrate
Legacy Pharmaceutical Packaging
Metoprolol Tartrate Tablets, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- METOPROLOL TARTRATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- METOPROLOL TARTRATE INDICATIONS AND USAGE
- METOPROLOL TARTRATE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- METOPROLOL TARTRATE ADVERSE REACTIONS
- OVERDOSAGE
- METOPROLOL TARTRATE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL
- PACKAGE LABEL PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
METOPROLOL TARTRATE DESCRIPTION
1pdextro
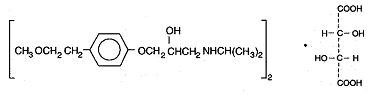
CLINICAL PHARMACOLOGY
In vitroin vivo12
121211
2
Pharmacokinetics
METOPROLOL TARTRATE INDICATIONS AND USAGE
Hypertension
Angina Pectoris
Myocardial Infarction
DOSAGE AND ADMINISTRATIONCONTRAINDICATIONSWARNINGSDOSAGE AND ADMINISTRATION
METOPROLOL TARTRATE CONTRAINDICATIONS
Hypertension and Angina
WARNINGS
WARNINGS
Myocardial Infarction
WARNINGS
WARNINGS
Hypertension and Angina
Cardiac Failure
In Patients Without a History of Cardiac Failure
Ischemic Heart Disease
Following abrupt cessation of therapy with certain beta-blocking agents, exacerbations of angina pectoris and, in some cases, myocardial infarction have occurred. When discontinuing chronically administered metoprolol, particularly in patients with ischemic heart disease, the dosage should be gradually reduced over a period of 1 to 2 weeks and the patient should be carefully monitored. If angina markedly worsens or acute coronary insufficiency develops, metoprolol administration should be reinstated promptly, at least temporarily, and other measures appropriate for the management of unstable angina should be taken. Patients should be warned against interruption or discontinuation of therapy without the physician's advice. Because coronary artery disease is common and may be unrecognized, it may be prudent not to discontinue metoprolol therapy abruptly even in patients treated only for hypertension.
Bronchospastic Diseases
PATIENTS WITH BRONCHOSPASTIC DISEASES SHOULD, IN GENERAL, NOT RECEIVE BETA-BLOCKERS, including metoprolol. Because of its relative beta1 selectivity, however, metoprolol may be used with caution in patients with bronchospastic disease who do not respond to, or cannot tolerate, other antihypertensive treatment. Since beta1 selectivity is not absolute, a beta2-stimulating agent should be administered concomitantly, and the lowest possible dose of metoprolol tartrate should be used. In these circumstances it would be prudent initially to administer metoprolol in smaller doses three times daily, instead of larger doses two times daily, to avoid the higher plasma levels associated with the longer dosing interval. (See DOSAGE AND ADMINISTRATION.)
Major Surgery
The necessity or desirability of withdrawing beta-blocking therapy, including metoprolol, prior to major surgery is controversial; the impaired ability of the heart to respond to reflex adrenergic stimuli may augment the risks of general anesthesia and surgical procedures.
Metoprolol, like other beta-blockers, is a competitive inhibitor of beta-receptor agonists, and its effects can be reversed by administration of such agents, e.g., dobutamine or isoproterenol. However, such patients may be subject to protracted severe hypotension. Difficulty in restarting and maintaining the heartbeat has also been reported with beta-blockers.
Diabetes and Hypoglycemia
Metoprolol should be used with caution in diabetic patients if a beta-blocking agent is required. Beta-blockers may mask tachycardia occurring with hypoglycemia, but other manifestations such as dizziness and sweating may not be significantly affected.
Pheochromocytoma
In patients known to have, or suspected of having, a pheochromocytoma, metoprolol is contraindicated (see CONTRAINDIATIONS). If metoprolol is required, it should be given in combination with an alpha blocker, and only after the alpha blocker has been initiated. Administration of beta-blockers alone in the setting of pheochromocytoma have been associated with a paradoxical increase in blood pressure due to the attenuation of beta-mediated vasodilatation in skeletal muscle.
Thyrotoxicosis
Beta-adrenergic blockade may mask certain clinical signs (e.g., tachycardia) of hyperthyroidism. Patients suspected of developing thyrotoxicosis should be managed carefully to avoid abrupt withdrawal of beta-blockade, which might precipitate a thyroid storm.
Myocardial Infarction
Cardiac Failure
Bradycardia
AV Block
Hypotension
Bronchospastic Diseases
PATIENTS WITH BRONCHOSPASTIC DISEASES SHOULD, IN GENERAL, NOT RECEIVE BETA-BLOCKERS, including metoprolol. Because of its relative beta1 selectivity, metoprolol may be used with extreme caution in patients with bronchospastic disease. Because it is unknown to what extent beta2-stimulating agents may exacerbate myocardial ischemia and the extent of infarction, these agents should not be used prophylactically. If bronchospasm not related to congestive heart failure occurs, metoprolol should be discontinued. A theophylline derivative or a beta2 agonist may be administered cautiously, depending on the clinical condition of the patient. Both theophylline derivatives and beta2 agonists may produce serious cardiac arrhythmias.
PRECAUTIONS
General
Information for Patients
Drug Interactions
General Anesthetics
WARNINGS, Major Surgery
CYP2D6 Inhibitors
Clonidine
Carcinogenesis, Mutagenesis, Impairment of Fertility
Pregnancy Category C
Nursing Mothers
Pediatric Use
Geriatric Use
METOPROLOL TARTRATE ADVERSE REACTIONS
Hypertension and Angina
Central Nervous System:
Cardiovascular: CONTRAINDICATIONSWARNINGSPRECAUTIONS
Respiratory: WARNINGS
Gastrointestinal:
Hypersensitive Reactions:
Miscellaneous:
Myocardial Infarction
Central Nervous System:
Cardiovascular: CLINICAL PHARMACOLOGY
| |
Metoprolol
|
Placebo
|
| Hypotension (systolic BP < 90 mm Hg) |
27.4% |
23.2% |
| Bradycardia (heart rate <40 beats/min) |
15.9% |
6.7% |
| Second- or third-degree heart block |
4.7% |
4.7% |
| First-degree heart block (P-R ≥0.26 sec) |
5.3% |
1.9% |
| Heart failure |
27.5% |
29.6% |
Respiratory:
Gastrointestinal:
Dermatologic:
Miscellaneous:
Potential Side Effects
Central Nervous System:
Cardiovascular: CONTRAINDICATIONS
Hematologic:
Hypersensitive Reactions:
Postmarketing Experience
OVERDOSAGE
Acute Toxicity:
50
Signs and Symptoms
Treatment
WARNINGS: Myocardial Infarction
Elimination of the Drug:
Bradycardia:
Hypotension:
Bronchospasm: 2
Cardiac Failure:
METOPROLOL TARTRATE DOSAGE AND ADMINISTRATION
Hypertension
1
Angina Pectoris
WARNINGS
Myocardial Infarction
Early Treatment
Late Treatment
WARNINGS
Late Treatment
HOW SUPPLIED
Metoprolol Tartrate Tablets, USP are available as follows:
Tablets 50 mg
are pink round shaped, film coated tablets debossed with ‘C over 74’ on one side and deep break line on other side.
Bottles of 60 NDC 68645-190-59
Tablets 100 mg
are light blue round shaped, film coated tablets debossed with ‘C over 75’ on one side and deep break line on other side.
Bottles of 60 NDC 68645-191-59
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Protect from moisture.
Dispense
Aurobindo Pharma Limited
Packaged by:
Legacy Pharmaceutical Packaging
Earth City, MO 63045
Distributed by:
Wal-Mart
Bentonville, AR 72716
Revised: 01/2009
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL
NDC 68645-190-59
Metoprolol
Tartrate
Tablets, USP
50 mg
Rx only
60 Tablets

PACKAGE LABEL PRINCIPAL DISPLAY PANEL
NDC 68645-191-59
Metoprolol
Tartrate
Tablets, USP
100mg
Rx Only
60 Tablets

Metoprolol TartrateMetoprolol Tartrate TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Metoprolol TartrateMetoprolol Tartrate TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||