Metronidazole
FULL PRESCRIBING INFORMATION: CONTENTS*
- USE IN SPECIFIC POPULATIONS
- WARNING
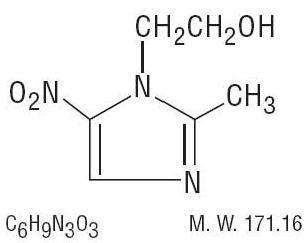
- METRONIDAZOLE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- METRONIDAZOLE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- METRONIDAZOLE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
USE IN SPECIFIC POPULATIONS
WARNING
Metronidazole has been shown to be carcinogenic in mice and rats. ( See PRECAUTIONS.) Unnecessary use of the drug should be avoided. Its use should be reserved for the conditions described in the INDICATIONS AND USAGEsection below.METRONIDAZOLE DESCRIPTION
CLINICAL PHARMACOLOGY
Microbiology
Susceptibility Tests
INDICATIONS & USAGE
Symptomatic TrichomoniasisAsymptomatic Trichomoniasis
Treatment of Asymptomatic Consorts
Amebiasis
Anaerobic Bacterial Infections
METRONIDAZOLE CONTRAINDICATIONS
WARNINGS.)
WARNINGS
Convulsive Seizures and Peripheral NeuropathyPRECAUTIONS
GeneralInformation for Patients
Drug Interactions).
Laboratory Tests
Metronidazole is a nitroimidazole and should be used with caution in patients with evidence of or history of blood dyscrasia. A mild leukopenia has been observed during its administration; however, no persistent hematologic abnormalities attributable to metronidazole have been observed in clinical studies. Total and differential leukocyte counts are recommended before and after therapy for trichomoniasis and amebiasis, especially if a second course of therapy is necessary, and before and after therapy for anaerobic infections.
Drug Interactions
Drug/Laboratory Test Interactions
Carcinogenesis, Mutagenesis, Impairment of Fertility
Pregnancy
Teratogenic Effects
Pregnancy category B
Metronidazole crosses the placental barrier and enters the fetal circulation rapidly. Reproduction studies have been performed in rats at doses up to five times the human dose and have revealed no evidence of impaired fertility or harm to the fetus due to metronidazole. No fetotoxicity was observed when metronidazole was administered orally to pregnant mice at 20 mg/kg/day approximately one and a half times the most frequently recommended human dose (750 mg/day) based on a mg/kg body weight; however in a single small study where the drug was administered intraperitoneally, some intrauterine deaths were observed. The relationship of these findings to the drug is unknown. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studeis are not always predictive of human response, and because metronidazole is a carcinogen in rodents, this drug should be used pregnancy only if clearly needed.
Nursing Mothers
Geriatric Use
Pediatric Use
METRONIDAZOLE ADVERSE REACTIONS
OVERDOSAGE
Treatment
There is no specific antidote for metronidazole overdose; therefore, management of the patient should consist of symptomatic and supportive therapy.
DOSAGE & ADMINISTRATION
Trichomoniasis
In the Female
CONTRAINDICATIONS.)In pregnant patients in whom alternative treatment has been inadequate, the one-day course of therapy should not be used, as it results in higher serum levels which can reach the fetal circulation (seePRECAUTIONS, Pregnancy).
In the Male
Amebiasis
Adults
Pediatric Patients
Anaerobic Bacterial Infections
HOW SUPPLIED
STORAGE AND HANDLING
REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

MetronidazoleMETRONIDAZOLE TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!