Mirtazapine
FULL PRESCRIBING INFORMATION: CONTENTS*
- Suicidality and Antidepressant Drugs
- MIRTAZAPINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- MIRTAZAPINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DRUG INTERACTIONS
- MIRTAZAPINE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- Medication Guide
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
Suicidality and Antidepressant Drugs
WARNINGS: Clinical Worsening and Suicide Risk, PRECAUTIONS: Information for Patients, and PRECAUTIONS: Pediatric Use)MIRTAZAPINE DESCRIPTION
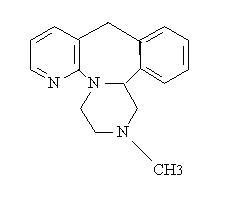
CLINICAL PHARMACOLOGY
PharmacodynamicsPharmacokinetics
Special Populations
Geriatric
Following oral administration of mirtazapine tablets 20 mg/day for 7 days to subjects of varying ages (range, 25 to 74), oral clearance of mirtazapine was reduced in the elderly compared to the younger subjects. The differences were most striking in males, with a 40% lower clearance in elderly males compared to younger males, while the clearance in elderly females was only 10% lower compared to younger females. Caution is indicated in administering mirtazapine tablets to elderly patients (seePRECAUTIONSandDOSAGE AND ADMINISTRATION).
Pediatrics
Safety and effectiveness of mirtazapine in the pediatric population have not been established (seePRECAUTIONS).
Gender
The mean elimination half-life of mirtazapine after oral administration ranges from approximately 20 to 40 hours across age and gender subgroups, with females of all ages exhibiting significantly longer elimination half-lives than males (mean half-life of 37 hours for females vs. 26 hours for males) (seePharmacokinetics)
Race
There have been no clinical studies to evaluate the effect of race on the pharmacokinetics of mirtazapine.
Renal Insufficiency
The disposition of mirtazapine was studied in patients with varying degrees of renal function. Elimination of mirtazapine is correlated with creatinine clearance. Total body clearance of mirtazapine was reduced approximately 30% in patients with moderate (Clcr = 11 to 39 mL/ min/1.73 m2) and approximately 50% in patients with severe (Clcr = < 10 mL/min/1.73 m2) renal impairment when compared to normal subjects. Caution is indicated in administering mirtazapine to patients with compromised renal function (seePRECAUTIONSandDOSAGE AND ADMINISTRATION).
Hepatic Insufficiency
Following a single 15 mg oral dose of mirtazapine, the oral clearance of mirtazapine was decreased by approximately 30% in hepatically impaired patients compared to subjects with normal hepatic function. Caution is indicated in administering mirtazapine to patients with compromised hepatic function (seePRECAUTIONSandDOSAGE AND ADMINISTRATION).
Clinical Trials Showing Effectiveness
INDICATIONS & USAGE
CLINICAL PHARMACOLOGY).
A major depressive episode (DSM-IV) implies a prominent and relatively persistent (nearly every day for at least 2 weeks) depressed or dysphoric mood that usually interferes with daily functioning, and includes at least 5 of the following 9 symptoms: depressed mood, loss of interest in usual activities, significant change in weight and/or appetite, insomnia or hypersomnia, psychomotor agitation or retardation, increased fatigue, feelings of guilt or worthlessness, slowed thinking or impaired concentration, a suicide attempt or suicidal ideation.
The effectiveness of mirtazapine in hospitalized depressed patients has not been adequately studied.
The efficacy of mirtazapine in maintaining a response in patients with major depressive disorder for up to 40 weeks following 8 to 12 weeks of initial open-label treatment was demonstrated in a placebo-controlled trial. Nevertheless, the physician who elects to use mirtazapine for extended periods should periodically re-evaluate the long-term usefulness of the drug for the individual patient (seeCLINICAL PHARMACOLOGY)
MIRTAZAPINE CONTRAINDICATIONS
HypersensitivityMonoamine Oxidase Inhibitors
WARNINGS,PRECAUTIONS: Drug Interactions, andDOSAGE AND ADMINISTRATION).
WARNINGS
Clinical Worsening and Suicide RiskTable 1
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to health care providers. Such monitoring should include daily observation by families and caregivers.Prescriptions for Mirtazapine tablets should be written for the smallest quantity of tablets consistent with good patient management, in order to reduce the risk of overdose.
Screening Patients for Bipolar Disorder:
A major depressive episode may be initial presentation of bipolar disorders. it is generally believed (though not established in controlled trials) tha treating such an epidose with an antidepressant alone may increase the likelihood of precipitation of a mixed/manic episode in patients at risk for bipolar disorder. Whether any of the symptoms decribed above represent such a conversion is unknown. However, prior to initiating treatment with an antidepressant, patients with depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression. It should be noted that mirtazapine tablets are not approved for use in treating bipolar depression.
Agranulocytosis
In premarketing clinical trials, 2 (1 with SjSyndrome) out of 2,796 patients treated with mirtazapine tablets developed agranulocytosis [absolute neutrophil count (ANC) < 500/mm3 with associated signs and symptoms, e.g., fever, infection, etc.] and a third patient developed severe neutropenia (ANC < 500/mm3 without any associated symptoms). For these 3 patients, onset of severe neutropenia was detected on days 61, 9, and 14 of treatment, respectively. All 3 patients recovered after mirtazapine was stopped. These 3 cases yield a crude incidence of severe neutropenia (with or without associated infection) of approximately 1.1 per thousand patients exposed, with a very wide 95% confidence interval i.e., 2.2 cases per 10,000 to 3.1 cases per 1,000. If a patient develops a sore throat, fever, stomatitis or other signs of infection, along with a low WBC count, treatment with mirtazapine should be discontinued and the patient should be closely monitored.
MAO Inhibitors
In patients receiving other drugs for major depressive disorder in combination with a monoamine oxidase inhibitor (MAOI) and in patients who have recently discontinued a drug for major depressive disorder and then are started on an MAOI, there have been reports of serious and sometimes fatal, reactions, including nausea, vomiting, flushing, dizziness, tremor, myoclonus, rigidity, diaphoresis, hyperthermia, autonomic instability with rapid fluctuations of vital signs, seizures, and mental status changes ranging from agitation to coma. Although there are no human data pertinent to such an interaction with mirtazapine tablets, it is recommended that mirtazapine not be used in combination with an MAOI, or within 14 days of initiating or discontinuing therapy with an MAOI.
Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-like Reactions
CONTRAINDICATIONS].
PRECAUTIONS
GeneralDiscontinuation Symptoms
There have been reports of adverse reactions upon the discontinuation of mirtazapine tablets (particularly when abrupt), including but not limited to the following: dizziness, abnormal dreams, sensory disturbances (including paresthesia and electric shock sensations), agitation, anxiety, fatigue, confusion, headache, tremor, nausea, vomiting, and sweating, or other symptoms which may be of clinical significance. The majority of the reported cases are mild and self-limiting. Even though these have been reported as adverse reactions, it should be realized that these symptoms may be related to underlying disease.
Patients currently taking mirtazapine should NOT discontinue treatment abruptly, due to risk of discontinuation symptoms. At the time that a medical decision is made to discontinue treatment with mirtazapine, a gradual reduction in the dose, rather than an abrupt cessation, is recommended.
Akathisia/Psychomotor Restlessness
The use of antidepressants has been associated with the development of akathisia, characterized by a subjectively unpleasant or distressing restlessness and need to move, often accompanied by an inability to sit or stand still. This is most likely to occur within the first few weeks of treatment. In patients who develop these symptoms, increasing the dose may be detrimental.
Hyponatremia
Hyponatremia has been reported very rarely with the use of mirtazapine. Caution should be exercised in patients at risk, such as elderly patients or patients concomitantly treated with medications known to cause hyponatremia.
Somnolence
In US controlled studies, somnolence was reported in 54% of patients treated with mirtazapine tablets, compared to 18% for placebo and 60% for amitriptyline. In these studies, somnolence resulted in discontinuation for 10.4% of mirtazapine treated patients, compared to 2.2% for placebo. It is unclear whether or not tolerance develops to the somnolent effects of mirtazapine. Because of the potentially significant effects of mirtazapine on impairment of performance, patients should be cautioned about engaging in activities requiring alertness until they have been able to assess the drugeffect on their own psychomotor performance (seeInformation for Patients).
Dizziness
In US controlled studies, dizziness was reported in 7% of patients treated with mirtazapine, compared to 3% for placebo and 14% for amitriptyline. It is unclear whether or not tolerance develops to the dizziness observed in association with the use of mirtazapine.
Increased Appetite/Weight Gain
In US controlled studies, appetite increase was reported in 17% of patients treated with mirtazapine, compared to 2% for placebo and 6% for amitryptyline. In these same trials, weight gain of7% of body weight was reported in 7.5% of patients treated with mirtazapine, compared to 0% for placebo and 5.9% for amitriptyline. In a pool of premarketing US studies, including many patients for long-term, open-label treatment, 8% of patients receiving mirtazapine discontinued for weight gain. In an 8-week-long pediatric clinical trial of doses between 15 to 45 mg/day, 49% of mirtazapine treated patients had a weight gain of at least 7%, compared to 5.7% of placebo-treated patients (seePRECAUTIONS: Pediatric Use).
Cholesterol/Triglycerides
In US controlled studies, nonfasting cholesterol increases to20% above the upper limits of normal were observed in 15% of patients treated with mirtazapine, compared to 7% for placebo and 8% for amitriptyline. In these same studies, nonfasting triglyceride increases to500 mg/dL were observed in 6% of patients treated with mirtazapine, compared to 3% for placebo and 3% for amitriptyline.
Transaminase Elevations
Clinically significant ALT (SGPT) elevations (3 times the upper limit of the normal range) were observed in 2% (8/424) of patients exposed to mirtazapine in a pool of short-term US controlled trials, compared to 0.3% (1/328) of placebo patients and 2% (3/181) of amitriptyline patients. Most of these patients with ALT increases did not develop signs or symptoms associated with compromised liver function. While some patients were discontinued for the ALT increases, in other cases, the enzyme levels returned to normal despite continued mirtazapine treatment. Mirtazapine should be used with caution in patients with impaired hepatic function (seeCLINICAL PHARMACOLOGYandDOSAGE AND ADMINISTRATION)
Activation of Mania/Hypomania
Mania/hypomania occurred in approximately 0.2% (3/1,299 patients) of mirtazapine treated patients in US studies. Although the incidence of mania/hypomania was very low during treatment with mirtazapine, it should be used carefully in patients with a history of mania/hypomania.
Seizure
In premarketing clinical trials only 1 seizure was reported among the 2,796 US and non-US patients treated with mirtazapine. However, no controlled studies have been carried out in patients with a history of seizures. Therefore, care should be exercised when mirtazapine is used in these patients.
Use in Patients with Concomitant Illness
Clinical experience with mirtazapine in patients with concomitant systemic illness is limited. Accordingly, care is advisable in prescribing mirtazapine for patients with diseases or conditions that affect metabolism or hemodynamic responses.
Mirtazapine has not been systematically evaluated or used to any appreciable extent in patients with a recent history of myocardial infarction or other significant heart disease. Mirtazapine was associated with significant orthostatic hypotension in early clinical pharmacology trials with normal volunteers. Orthostatic hypotension was infrequently observed in clinical trials with depressed patients. Mirtazapine should be used with caution in patients with known cardiovascular or cerebrovascular disease that could be exacerbated by hypotension (history of myocardial infarction, angina, or ischemic stroke) and conditions that would predispose patients to hypotension (dehydration, hypovolemia, and treatment with antihypertensive medication).
Mirtazapine clearance is decreased in patients with moderate [glomerular filtration rate (GFR) = 11 to 39 mL/min/1.73 m2] and severe [GFR < 10 mL/min/1.73 m2] renal impairment, and also in patients with hepatic impairment. Caution is indicated in administering mirtazapine to such patients (seeCLINICAL PHARMACOLOGYandDOSAGE AND ADMINISTRATION)
Information for Patients
Clinical Worsening and Suicide Risk
Patients, their families, and their caregivers should be encouraged to be alert to the emergence of anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, mania, other unusual changes in behavior, worsening of depression, and suicidal ideation, especially early during antidepressant treatment and when the dose is adjusted up or down. Families and caregivers of patients should be advised to look for the emergence of such symptoms on a day-to-day basis, since changes may be abrupt. Such symptoms should be reported to the patient's prescriber or health professional, especially if they are severe, abrupt in onset, or were not part of the patient's presenting symptoms. Symptoms such as these may be associated with an increased risk for suicidal thinking and behavior and indicate a need for very close monitoring and possibly changes in the medication.
Agranulocytosis
Interference with Cognitive and Motor Performance
Mirtazapine may impair judgement, thinking and particularly, motor skills, because of its prominent sedative effect. The drowsiness associated with mirtazapine use may impair a patientability to drive, use machines or perform tasks that require alertness. Thus, patients should be cautioned about engaging in hazardous activities until they are reasonably certain that mirtazapine therapy does not adversely affect their ability to engage in such activities.
Completing Course of Therapy
While patients may notice improvement with mirtazapine therapy in 1 to 4 weeks, they should be advised to continue therapy as directed.
Concomitant Medication
Patients should be advised to inform their physician if they are taking, or intend to take, any prescription or over-the-counter drugs since there is a potential for mirtazapine to interact with other drugs.
Alcohol
The impairment of cognitive and motor skills produced by mirtazapine has been shown to be additive with those produced by alcohol. Accordingly, patients should be advised to avoid alcohol while taking mirtazapine.
Pregnancy
Patients should be advised to notify their physician if they become pregnant or intend to become pregnant during mirtazapine therapy.
Nursing
Patients should be advised to notify their physician if they are breast-feeding an infant.
Laboratory Tests
DRUG INTERACTIONS
CLINICAL PHARMACOLOGY).
Monoamine Oxidase Inhibitors
(SeeCONTRAINDICATIONS,WARNINGS, andDOSAGE AND ADMINISTRATION.)
Serotonergic Drugs
Based on the mechanism of action of mirtazapine and the potential for serotonin syndrome, caution is advised when mirtazapine tablets are coadministered with other drugs or agents that may affect the serotonergic neurotransmitter systems, such as tryptophan, triptans, linezolid, serotonin reuptake inhibitors, venlafaxine, lithium, tramadol, or St. John's wort (seeCONTRAINDICATIONSandWARNINGS).
Drugs Affecting Hepatic Metabolism
The metabolism and pharmacokinetics of mirtazapine tablets may be affected by the induction or inhibition of drug-metabolizing enzymes.
Drugs that are Metabolized by and/or Inhibit Cytochrome P450 Enzymes
CYP Enzyme Inducers(these studies used both drugs at steady state)
Phenytoin
In healthy male patients (n=18), phenytoin (200 mg daily) increased mirtazapine (30 mg daily) clearance about 2-fold, resulting in a decrease in average plasma mirtazapine concentrations of 45%. Mirtazapine did not significantly affect the pharmacokinetics of phenytoin.
Carbamazepine
In healthy male patients (n=24), carbamazepine (400 mg b.i.d.) increased mirtazapine (15 mg b.i.d.) clearance about 2-fold, resulting in a decrease in average plasma mirtazapine concentrations of 60%.
When phenytoin, carbamazepine, or another inducer of hepatic metabolism (such as rifampicin) is added to mirtazapine therapy, the mirtazapine dose may have to be increased. If treatment with such a medicinal product is discontinued, it may be necessary to reduce the mirtazapine dose.
CYP Enzyme Inhibitors
Cimetidine
In healthy male patients (n=12), when cimetidine, a weak inhibitor of CYP1A2, CYP2D6, and CYP3A4, given at 800 mg b.i.d. at steady state was coadministered with mirtazapine (30 mg daily) at steady state, the Area Under the Curve (AUC) of mirtazapine increased more than 50%. Mirtazapine did not cause relevant changes in the pharmacokinetics of cimetidine. The mirtazapine dose may have to be decreased when concomitant treatment with cimetidine is started, or increased when cimetidine treatment is discontinued.
Ketoconazole
In healthy, male, Caucasian patients (n=24), coadministration of the potent CYP3A4 inhibitor ketoconazole (200 mg b.i.d. for 6.5 days) increased the peak plasma levels and the AUC of a single 30 mg dose of mirtazapine by approximately 40% and 50%, respectively.
Caution should be exercised when coadministering mirtazapine with potent CYP3A4 inhibitors, HIV protease inhibitors, azole antifungals, erythromycin, or nefazodone
Paroxetine
In an in vivo interaction study in healthy, CYP2D6 extensive metabolizer patients (n=24), mirtazapine (30 mg/day), at steady state, did not cause relevant changes in the pharmacokinetics of steady state paroxetine (40 mg/day), a CYP2D6 inhibitor.
Other Drug-Drug Interactions
Amitriptyline
In healthy, CYP2D6 extensive metabolizer patients (n=32), amitriptyline (75 mg daily), at steady state, did not cause relevant changes in the pharmacokinetics of steady state mirtazapine (30 mg daily); mirtazapine also did not cause relevant changes to the pharmacokinetics of amitriptyline.
Warfarin
In healthy male subjects (n=16), mirtazapine (30 mg daily), at steady state, caused a small (0.2) but statistically significant increase in the International Normalized Ratio (INR) in subjects treated with warfarin. As at a higher dose of mirtazapine, a more pronounced effect can not be excluded. It is advisable to monitor the INR in case of concomitant treatment of warfarin with mirtazapine.
Lithium
No relevant clinical effects or significant changes in pharmacokinetics have been observed in healthy male subjects on concurrent treatment with subtherapeutic levels of lithium (600 mg/day for 10 days) at steady state and a single 30 mg dose of mirtazapine. The effects of higher doses of lithium on the pharmacokinetics of mirtazapine are unknown.
Risperidone
In an in vivo, nonrandomized, interaction study, subjects (n=6) in need of treatment with an antipsychotic and antidepressant drug, showed that mirtazapine (30 mg daily) at steady state did not influence the pharmacokinetics of risperidone (up to 3 mg b.i.d.).
Alcohol
Concomitant administration of alcohol (equivalent to 60 g) had a minimal effect on plasma levels of mirtazapine (15 mg) in 6 healthy male subjects. However, the impairment of cognitive and motor skills produced by mirtazapine were shown to be additive with those produced by alcohol. Accordingly, patients should be advised to avoid alcohol while taking mirtazapine.
Diazepam
Concomitant administration of diazepam (15 mg) had a minimal effect on plasma levels of mirtazapine (15 mg) in 12 healthy subjects. However, the impairment of motor skills produced by mirtazapine has been shown to be additive with those caused by diazepam. Accordingly, patients should be advised to avoid diazepam and other similar drugs while taking mirtazapine.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Pregnancy
Teratogenic EffectsPregnancy Category C
Reproduction studies in pregnant rats and rabbits at doses up to 100 mg/kg and 40 mg/kg, respectively [20 and 17 times the maximum recommended human dose (MRHD) on an mg/m2 basis, respectively], have revealed no evidence of teratogenic effects. However, in rats, there was an increase in postimplantation losses in dams treated with mirtazapine. There was an increase in pup deaths during the first 3 days of lactation and a decrease in pup birth weights. The cause of these deaths is not known. The effects occurred at doses that were 20 times the MRHD, but not at 3 times the MRHD, on an mg/m2 basis. There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Pediatric Use
BOX WARNING and WARNINGSWorsening and Suicide Risk). Two placebo-controlled trials in 258 pediatric patients with MDD have been conducted with mirtazapine tablets, and the data were not sufficient to support a claim for use in pediatric patients. Anyone considering the use of mirtazapine tablets in a child or adolescent must balance the potential risks with the clinical need.
PRECAUTIONS: Increased Appetite/Weight Gain).
Geriatric Use
CLINICAL PHARMACOLOGYandDOSAGE AND ADMINISTRATION).
MIRTAZAPINE ADVERSE REACTIONS
Associated with Discontinuation of TreatmentCommonly Observed Adverse Events in US Controlled Clinical Trials
Table 3: Common TreatmentAdverse Events Associated with the Use of Mirtazapine
in 6-Week US Trials
Adverse Events Occurring at an Incidence of 1% or More Among Mirtazapine-Treated Patients
Table 4: Incidence of Adverse Clinical Experiences1 (In Short-Term U.S. Controlled Studies
Body System Adverse Clinical Experience Mirtazapine
(n=453) Placebo
(n=361)
ECG Changes
Other Adverse Events Observed During the Premarketing Evaluation of Mirtazapine
WARNINGSandPRECAUTIONSsections.
Events are further categorized by body system and listed in order of decreasing frequent according to the following definitions: frequent adverse events are those occurring on 1 or more occasions in at least 1/100 patients; infrequent adverse events are those occurring in 1/100 to 1/1000 patients; rare events are those occurring in fewer than 1/1000 patients. Only those events not already listed in Table 4 appear in this listing. Events of major clinical impotance are also described in the WARNINGS and PRECAUTIONS sections.
Body as a Whole:
frequent:malaise, abdominal pain, abdominal syndrome acute; infrequent:chills, fever, face edema, ulcer, photosensitivity reaction, neck rigidity, neck pain, adomen enlarged; rare: cellulitis, chest pain substernal.
Cardiovascular System:
frequent:hypertension, vasodilatation; infrequent;angina pectois, myocardial infarction, bradycardia, ventricular extrasystoles, syncope, migraine, hypotension;rare:atrial arrhythmia, bigeminy, vascular headache, pulmonary embolus, cerebral ischemia, cardiomegaly, phlebitis, left heart failure.
Digestive System:
frequent:vomiting, anorexia;infrequent:eructation, glossitis, cholecystitis, nausea and vomiting, gum hemorrhage, stomatitis, colitis, liver function tests abnormal; rare: tongue discoloration, ulcerative stomatitis, salivary gland enlargement, increased salivation, intestinal obstruction, pancreatitis, aphthous stomatitis, cirrhosis of liver, gastritis, gastroenteritis, oral moniliasis, tongue edema.
Endocrine System:rare: goiter, hypothyroidism.
Hemic and Lymphatic System:rare: lymphadenopathy, leukopenia, petechia, anemia, thrombocytopenia, lymphocytosis, pancytopenia.
Metabolic and Nutritional Disorders:
frequent:thirst; infrequent:dehydration, weight loss; rare:gout, SGOT increased, healing abnormal, acid phosphatase increased, SGPT increased, diabetes melllitus, hyponatremia.
Musculoskeletal System:
frequent:myasthenia, arthralgia; infrequent: arthritis, tenosynovitis;rare: pathologic fracture, osteoporosis fracture, bone pain, myositis, tendon rupture, arthrosis, bursitis.
Nervous System:
frequent:hypesthesia, apathy, depression, hypokinesia, vertigo, twitching, agitatiom, anxiety, amnesia, hyperkinesia, paresthesia; infrequent:ataxia, delirium, delusions, depersonalization, dyskinesia, extrapyramidal syndrome, libido increased, coordination abnormal, dysarthria, hallucinations, manic reaction, neurosis, dystonia, hostility, reflexes increased, emotional lability, euphoria, paranoid reaction; rare: aphasia, nystagmus, akathisia (psychomotor restlessness), stupor, dementia, diplopia, drug dependence, paralysis, grand mal convulsion, hypotonia, myoclonus, psychotic depression, withdrawal syndrome, serotonin syndrome.
Respiratory System:
frequent: cough increased, sinusitis; infrequent: epistaxis, bronchitis, asthma, pneuomnia; rare:asphyxia, laryngitis, pneumothorax, hiccup.
Skin and Appendages:
frequent: oruritus, rash;infrequent: acne, exfoliative dermatitis, dry skin, herpes simplex, alopecia; rare: urticaria, herpes zoster, skin hypertrophy, seborrhea, skin ulcer.
Special Senses:
infrequent:eye pain, abnormality of accommodation, conjunctivitis, deafness, keratoconjunctivitis, lacrimation disorder, glaucoma, hyperacusis, ear pain; rare:blepharitis, parital transitory deafness, otitis media, taste loss, parosmia.
Urogenital System:
frequent: urinary tract infection; infrequent:kidney calculus, cystitis, dysuria, urinary incontinence, urinary retention, vaginitis, hematuria, breast pain, amenorrhea, dysmenorrhea, leukorrhea, impotence;rare:polyuria, urethritis, metrorrhagia, menorrhagia, abnormal ejaculation, breast engorgement, breast enlargment, urinary urgency.
Other Adverse Events Observed During Postmarketing Evaluation of Mirtazapine
DRUG ABUSE AND DEPENDENCE
Controlled Substance ClassPhysical and Psychological Dependence
OVERDOSAGE
Human ExperienceOverdose Management
DOSAGE & ADMINISTRATION
Initial TreatmentThe recommended starting dose for mirtazapine tablets is 15 mg/day, administered in a single dose, preferably in the evening prior to sleep. In the controlled clinical trials establishing the efficacy of mirtazapine in the treatment of major depressive disorder, the effective dose range was generally 15 to 45 mg/day. While the relationship between dose and satisfactory response in the treatment of major depressive disorder for mirtazapine has not been adequately explored, patients not responding to the initial 15 mg dose may benefit from dose increases up to a maximum of 45 mg/day. Mirtazapine has an elimination half-life of approximately 20 to 40 hours; therefore, dose changes should not be made at intervals of less than 1 to 2 weeks in order to allow suddicient time for evaluation of the therapeutic response to a given dose.
Elderly and Patients with Renal or Hepatic Impairment
PRECAUTIONSandCLINICAL PHARMACOLOGY).
Maintenance/Extended Treatment
CLINICAL PHARMACOLOGY). Based on these limited data, it is unknown whether or not the dose of mirtazapine needed for maintenance treatment is identical to the dose needed to achieve an initial response. Patients should be periodically reassessed to determine the need for maintenance treatment and the appropriate dose for such treatment.
Switching Patients To or From a Monoamine Oxidase Inhibitor
Discontinuation of Mirtazapine Tablets treatment
PRECAUTIONSandADVERSE REACTIONS).
HOW SUPPLIED
7.5 mg TabletsWhite, biconvex, capsule shaped film coated tablets withdebossed on one side anddebossed on the other side.
Bottles of 30 NDC 13107-001-30
Bottles of 60 NDC 13107-001-60
Bottles of 90 NDC 13107-001-90
Bottles of 100 NDC 13107-001-01
Bottles of 500 NDC 13107-001-05
15 mg TabletsYellow, biconvex, capsule shaped film coated tablets with a score line in betweenandon one side anddebossed on the other side.
Bottles of 30 (120cc container) NDC 13107-031-30
Bottles of 30(30cc container) NDC 13107-031-34
Bottles of 60 NDC 13107-031-60
Bottles of 90 NDC 13107-031-90
Bottles of 100 NDC 13107-031-01
Bottles of 500 NDC 13107-031-05
30 Unit-of-use packaging NDC 13107-031-32
30 mg TabletsReddish Brown, biconvex, capsule shaped film coated tablets with a score line in betweenandon one side anddebossed on the other side.
Bottles of 30 (120cc container) NDC 13107-003-30
Bottles of 30(30cc container) NDC 13107-003-34
Bottles of 60 NDC 13107-003-60
Bottles of 90 NDC 13107-003-90
Bottles of 100 NDC 13107-003-01
Bottles of 500 NDC 13107-003-05
30 Unit-of-use packaging NDC 13107-003-32
45 mg TabletsWhite, biconvex, capsule shaped film coated tablets withdebossed on one side anddebossed on the other side.
Bottles of 30(30cc container) NDC 13107-032-34
Bottles of 60 NDC 13107-032-60
Bottles of 90 NDC 13107-032-90
Bottles of 100 NDC 13107-032-01
Bottles of 500 NDC 13107-032-05
30 Unit-of-use packaging NDC 13107-032-32
STORAGE AND HANDLING
Store at25(77to 30(59to 86[see USP Controlled Room Temperature]. Protect from light and moisture.Medication Guide
Mirtazapine Tablets, USPRead the Medication Guide that comes with mirtazapine tablets before you start taking them and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking to your healthcare provider about your medical condition or treatment. If you have any questions about mirtazapine tablets, talk to your healthcare provider.
What is the most important information I should know about mirtazapine tablets?
1. Suicidal thoughts or actions:
Mirtazapine tablets and other antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, or young adults within the first few months of treatment or when the dose is changed.
Call your healthcare provider right away if you have any of the following symptoms, or call 911 if an emergency, especially if they are new, worse, or worry you:
Call your healthcare provider right away if you have any of the following symptoms, or call 911 if an emergency. Mirtazapine tablets may be associated with these serious side effects:
2. Manic episodes:
3.Decreased White Blood Cellscalled neutrophils, which are needed to fight infections. Tell your doctor if you have any indication of infection such as fever, chills, sore throat, or mouth or nose sores, especially symptoms which are flu-like.
4. Serotonin Syndrome or Neuroleptic Malignant Syndrome-like reactions. This condition can be life-threatening and may include:
5. Seizures
6. Low salt (sodium) levels in the blood.Elderly people may be at greater risk for this. Symptoms may include:
7. Sleepiness.It is best to take mirtazapine tablets close to bedtime.
8. Severe skin reactions:Call your doctor right away if you have any or all of the following symptoms:
9. Severe allergic reactions: trouble breathing, swelling of the face, tongue, eyes or mouth
10. Increases in appetite or weight.Children and adolescents should have height and weight monitored during treatment.
11. Increased cholesterol and triglyceride levels in your blood
Do not stop mirtazapine tablets without first talking to your healthcare provider.Stopping mirtazapine tablets too quickly may cause potentially serious symptoms including:
What are mirtazapine tablets?
Who should not take mirtazapine tablets?
People who take mirtazapine tablets close in time to an MAOI may have serious or even life-threatening side effects. Get medical help right away if you have any of these symptoms:
What should I tell my healthcare provider before taking mirtazapine tablets? Ask if you are not sure.
Tell your healthcare provider about all the medicines that you take,including prescription and non-prescription medicines, vitamins, and herbal supplements. Mirtazapine tablets and some medicines may interact with each other, may not work as well, or may cause serious side effects.
How should I take mirtazapine tablets?
What should I avoid while taking mirtazapine tablets?
What are the possible side effects of mirtazapine tablets?
"What is the most important information I should know about mirtazapine tablets?"
CALL YOUR DOCTOR FOR MEDICAL ADVICE ABOUT SIDE EFFECTS. YOU MAY REPORT SIDE EFFECTS TO THE FDA AT 1-800-FDA-1088.
How should I store mirtazapine tablets?
Store at25(77to 30(59to 86[see USP Controlled Room Temperature].
Keep mirtazapine tablets and all medicines out of the reach of children.
General information about mirtazapine tablets
What are the ingredients in mirtazapine tablets?
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION


MirtazapineMirtazapine TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!