Mirtazapine
MIRTAZAPINE TABLETS
FULL PRESCRIBING INFORMATION: CONTENTS*
- MIRTAZAPINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- MIRTAZAPINE INDICATIONS AND USAGE
- MIRTAZAPINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- MIRTAZAPINE ADVERSE REACTIONS
- Associated with Discontinuation of Treatment
- Commonly Observed Adverse Events in U.S. Controlled Clinical Trials
- Adverse Events Occurring at an Incidence of 1% or More Among Mirtazapine-Treated Patients
- ECG Changes
- Other Adverse Events Observed During the Premarketing Evaluation of Mirtazapine
- Other Adverse Events Observed During Postmarketing Evaluation of Mirtazapine
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- MIRTAZAPINE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
FULL PRESCRIBING INFORMATION
Rx only
Suicidality and Antidepressant Drugs
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of mirtazapine or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Mirtazapine is not approved for use in pediatric patients. (See WARNINGS: Clinical Worsening and Suicide Risk, PRECAUTIONS: Information for Patients, and PRECAUTIONS: Pediatric Use)
MIRTAZAPINE DESCRIPTION
Mirtazapine tablets are an orally administered drug. Mirtazapine has a tetracyclic chemical structure and belongs to the piperazino-azepine group of compounds. It is designated 1,2,3,4,10,14b - hexahydro - 2 - methylpyrazino [2,1-a] pyrido [2,3-c] benzazepine and has the molecular formula of C17H19N3. Its molecular weight is 265.36. The structural formula is the following and it is the racemic mixture:
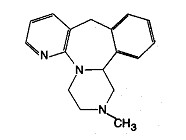
Mirtazapine is a white to creamy white crystalline powder which is slightly soluble in water.
Mirtazapine tablets are supplied for oral administration as scored film-coated tablets containing 15 mg or 30 mg of mirtazapine, and unscored film-coated tablets containing 45 mg of mirtazapine. Each tablet also contains the following inactive ingredients: anhydrous lactose, colloidal silicon dioxide, hydroxypropyl cellulose, hypromellose, iron oxide black (30 mg), iron oxide red (30 mg), iron oxide yellow (15 and 30 mg), lactose monohydrate, magnesium stearate, maize (corn) starch, polyethylene glycol, titanium dioxide and triacetin (15 mg).
CLINICAL PHARMACOLOGY
Pharmacodynamics
The mechanism of action of mirtazapine, as with other drugs effective in the treatment of major depressive disorder, is unknown.
Evidence gathered in preclinical studies suggests that mirtazapine enhances central noradrenergic and serotonergic activity. These studies have shown that mirtazapine acts as an antagonist at central presynaptic α2 adrenergic inhibitory autoreceptors and heteroreceptors, an action that is postulated to result in an increase in central noradrenergic and serotonergic activity.
Mirtazapine is a potent antagonist of 5-HT2 and 5-HT3 receptors. Mirtazapine has no significant affinity for the 5-HT1A and 5-HT1B receptors.
Mirtazapine is a potent antagonist of histamine (H1) receptors, a property that may explain its prominent sedative effects.
Mirtazapine is a moderate peripheral α1 adrenergic antagonist, a property that may explain the occasional orthostatic hypotension reported in association with its use.
Mirtazapine is a moderate antagonist at muscarinic receptors, a property that may explain the relatively low incidence of anticholinergic side effects associated with its use.
Pharmacokinetics
Mirtazapine is rapidly and completely absorbed following oral administration and has a half-life of about 20 to 40 hours. Peak plasma concentrations are reached within about 2 hours following an oral dose. The presence of food in the stomach has a minimal effect on both the rate and extent of absorption and does not require a dosage adjustment.
Mirtazapine is extensively metabolized after oral administration. Major pathways of biotransformation are demethylation and hydroxylation followed by glucuronide conjugation. In vitro data from human liver microsomes indicate that cytochrome 2D6 and 1A2 are involved in the formation of the 8-hydroxy metabolite of mirtazapine, whereas cytochrome 3A is considered to be responsible for the formation of the N-desmethyl and N-oxide metabolite. Mirtazapine has an absolute bioavailability of about 50%. It is eliminated predominantly via urine (75%) with 15% in feces. Several unconjugated metabolites possess pharmacological activity but are present in the plasma at very low levels. The (-) enantiomer has an elimination half-life that is approximately twice as long as the (+) enantiomer and therefore achieves plasma levels that are about three times as high as that of the (+) enantiomer.
Plasma levels are linearly related to dose over a dose range of 15 to 80 mg. The mean elimination half-life of mirtazapine after oral administration ranges from approximately 20 to 40 hours across age and gender subgroups, with females of all ages exhibiting significantly longer elimination half-lives than males (mean half-life of 37 hours for females versus 26 hours for males). Steady state plasma levels of mirtazapine are attained within 5 days, with about 50% accumulation (accumulation ratio = 1.5).
Mirtazapine is approximately 85% bound to plasma proteins over a concentration range of 0.01 to 10 µg/mL.
Special Populations
Geriatric
Following oral administration of mirtazapine 20 mg/day for 7 days to subjects of varying ages (range, 25 to 74), oral clearance of mirtazapine was reduced in the elderly compared to the younger subjects. The differences were most striking in males, with a 40% lower clearance in elderly males compared to younger males, while the clearance in elderly females was only 10% lower compared to younger females. Caution is indicated in administering mirtazapine to elderly patients (see PRECAUTIONS and DOSAGE AND ADMINISTRATION).
Pediatrics
Safety and effectiveness of mirtazapine in the pediatric population have not been established (see PRECAUTIONS).
Gender
The mean elimination half-life of mirtazapine after oral administration ranges from approximately 20 to 40 hours across age and gender subgroups, with females of all ages exhibiting significantly longer elimination half-lives than males (mean half-life of 37 hours for females versus 26 hours for males) (see Pharmacokinetics).
Race
There have been no clinical studies to evaluate the effect of race on the pharmacokinetics of mirtazapine.
Renal Insufficiency
The disposition of mirtazapine was studied in patients with varying degrees of renal function. Elimination of mirtazapine is correlated with creatinine clearance. Total body clearance of mirtazapine was reduced approximately 30% in patients with moderate (Clcr = 11 to 39 mL/min/1.73 m2) and approximately 50% in patients with severe (Clcr = <10 mL/min/1.73 m2) renal impairment when compared to normal subjects. Caution is indicated in administering mirtazapine to patients with compromised renal function (see PRECAUTIONS and DOSAGE AND ADMINISTRATION).
Hepatic Insufficiency
Following a single 15 mg oral dose of mirtazapine, the oral clearance of mirtazapine was decreased by approximately 30% in hepatically impaired patients compared to subjects with normal hepatic function. Caution is indicated in administering mirtazapine to patients with compromised hepatic function (see PRECAUTIONS and DOSAGE AND ADMINISTRATION).
Clinical Trials Showing Effectiveness
The efficacy of mirtazapine as a treatment for major depressive disorder was established in four placebo-controlled, 6-week trials in adult outpatients meeting DSM-III criteria for major depressive disorder. Patients were titrated with mirtazapine from a dose range of 5 mg up to 35 mg/day. Overall, these studies demonstrated mirtazapine to be superior to placebo on at least three of the following four measures: 21-Item Hamilton Depression Rating Scale (HDRS) total score; HDRS Depressed Mood Item; CGI Severity score; and Montgomery and Asberg Depression Rating Scale (MADRS). Superiority of mirtazapine over placebo was also found for certain factors of the HDRS, including anxiety/somatization factor and sleep disturbance factor. The mean mirtazapine dose for patients who completed these four studies ranged from 21 to 32 mg/day. A fifth study of similar design utilized a higher dose (up to 50 mg) per day and also showed effectiveness.
Examination of age and gender subsets of the population did not reveal any differential responsiveness on the basis of these subgroupings.
In a longer-term study, patients meeting (DSM-IV) criteria for major depressive disorder who had responded during an initial 8 to 12 weeks of acute treatment on mirtazapine were randomized to continuation of mirtazapine or placebo for up to 40 weeks of observation for relapse. Response during the open phase was defined as having achieved a HAM-D 17 total score of ≤8 and a CGI - Improvement score of 1 or 2 at two consecutive visits beginning with week 6 of the 8 to 12 weeks in the open-label phase of the study. Relapse during the double-blind phase was determined by the individual investigators. Patients receiving continued mirtazapine treatment experienced significantly lower relapse rates over the subsequent 40 weeks compared to those receiving placebo. This pattern was demonstrated in both male and female patients.
MIRTAZAPINE INDICATIONS AND USAGE
Mirtazapine tablets are indicated for the treatment of major depressive disorder.
The efficacy of mirtazapine in the treatment of major depressive disorder was established in six week controlled trials of outpatients whose diagnoses corresponded most closely to the Diagnostic and Statistical Manual of Mental Disorders - 3rd edition (DSM-III) category of major depressive disorder (see CLINICAL PHARMACOLOGY).
A major depressive episode (DSM-IV) implies a prominent and relatively persistent (nearly every day for at least 2 weeks) depressed or dysphoric mood that usually interferes with daily functioning, and includes at least five of the following nine symptoms: depressed mood, loss of interest in usual activities, significant change in weight and/or appetite, insomnia or hypersomnia, psychomotor agitation or retardation, increased fatigue, feelings of guilt or worthlessness, slowed thinking or impaired concentration, a suicide attempt or suicidal ideation.
The effectiveness of mirtazapine in hospitalized depressed patients has not been adequately studied.
The efficacy of mirtazapine in maintaining a response in patients with major depressive disorder for up to 40 weeks following 8 to 12 weeks of initial open-label treatment was demonstrated in a placebo-controlled trial. Nevertheless, the physician who elects to use mirtazapine for extended periods should periodically re-evaluate the long-term usefulness of the drug for the individual patient (see CLINICAL PHARMACOLOGY).
MIRTAZAPINE CONTRAINDICATIONS
Mirtazapine tablets are contraindicated in patients with a known hypersensitivity to mirtazapine.
WARNINGS
Clinical Worsening and Suicide Risk
Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a longstanding concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment. Pooled analyses of short-term placebo-controlled trials of antidepressant drugs (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18 to 24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
The pooled analyses of placebo-controlled trials in children and adolescents with MDD, obsessive compulsive disorder (OCD), or other psychiatric disorders included a total of 24 short-term trials of 9 antidepressant drugs in over 4400 patients. The pooled analyses of placebo-controlled trials in adults with MDD or other psychiatric disorders included a total of 295 short-term trials (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs placebo), however, were relatively stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1000 patients treated) are provided in Table 1.
| Table 1 | |
| Age Range | Drug-Placebo Difference in Number of Cases of Suicidality per 1000 Patients Treated |
| Drug-Related Increases | |
| <18 | 14 additional cases |
| 18-24 | 5 additional cases |
| Drug-Related Decreases | |
| 25-64 | 1 fewer case |
| >65 | 6 fewer cases |
No suicides occurred in any of the pediatric trials. There were suicides in the adult trials, but the number was not sufficient to reach any conclusion about drug effect on suicide.
It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the use of antidepressants can delay the recurrence of depression.
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patient's presenting symptoms.
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to healthcare providers. Such monitoring should include daily observation by families and caregivers. Prescriptions for mirtazapine should be written for the smallest quantity of tablets consistent with good patient management, in order to reduce the risk of overdose.
Screening Patients for Bipolar Disorder: A major depressive episode may be the initial presentation of bipolar disorder. It is generally believed (though not established in controlled trials) that treating such an episode with an antidepressant alone may increase the likelihood of precipitation of a mixed/manic episode in patients at risk for bipolar disorder. Whether any of the symptoms described above represent such a conversion is unknown. However, prior to initiating treatment with an antidepressant, patients with depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression. It should be noted that mirtazapine is not approved for use in treating bipolar depression.
Agranulocytosis
In premarketing clinical trials, two (one with Sjögren’s Syndrome) out of 2,796 patients treated with mirtazapine developed agranulocytosis (absolute neutrophil count (ANC) <500/mm3 with associated signs and symptoms, e.g., fever, infection, etc.) and a third patient developed severe neutropenia (ANC <500/mm3 without any associated symptoms). For these three patients, onset of severe neutropenia was detected on days 61, 9, and 14 of treatment, respectively. All three patients recovered after mirtazapine was stopped. These three cases yield a crude incidence of severe neutropenia (with or without associated infection) of approximately 1.1 per thousand patients exposed, with a very wide 95% confidence interval, i.e., 2.2 cases per 10,000 to 3.1 cases per 1000. If a patient develops a sore throat, fever, stomatitis or other signs of infection, along with a low WBC count, treatment with mirtazapine should be discontinued and the patient should be closely monitored.
MAO Inhibitors
In patients receiving other drugs for major depressive disorder in combination with a monoamine oxidase inhibitor (MAOI) and in patients who have recently discontinued a drug for major depressive disorder and then are started on an MAOI, there have been reports of serious, and sometimes fatal, reactions, e.g., including nausea, vomiting, flushing, dizziness, tremor, myoclonus, rigidity, diaphoresis, hyperthermia, autonomic instability with rapid fluctuations of vital signs, seizures, and mental status changes ranging from agitation to coma. Although there are no human data pertinent to such an interaction with mirtazapine, it is recommended that mirtazapine not be used in combination with an MAOI, or within 14 days of initiating or discontinuing therapy with an MAOI.
PRECAUTIONS
General
Somnolence
In U.S. controlled studies, somnolence was reported in 54% of patients treated with mirtazapine, compared to 18% for placebo and 60% for amitriptyline. In these studies, somnolence resulted in discontinuation for 10.4% of mirtazapine-treated patients, compared to 2.2% for placebo. It is unclear whether or not tolerance develops to the somnolent effects of mirtazapine. Because of mirtazapine’s potentially significant effects on impairment of performance, patients should be cautioned about engaging in activities requiring alertness until they have been able to assess the drug’s effect on their own psychomotor performance (see Information for Patients).
Dizziness
In U.S. controlled studies, dizziness was reported in 7% of patients treated with mirtazapine, compared to 3% for placebo and 14% for amitriptyline. It is unclear whether or not tolerance develops to the dizziness observed in association with the use of mirtazapine.
Increased Appetite/Weight Gain
In U.S. controlled studies, appetite increase was reported in 17% of patients treated with mirtazapine, compared to 2% for placebo and 6% for amitriptyline. In these same trials, weight gain of ≥7% of body weight was reported in 7.5% of patients treated with mirtazapine, compared to 0% for placebo and 5.9% for amitriptyline. In a pool of premarketing U.S. studies, including many patients for long-term, open label treatment, 8% of patients receiving mirtazapine discontinued for weight gain.
Cholesterol/Triglycerides
In U.S. controlled studies, nonfasting cholesterol increases to ≥ 20% above the upper limits of normal were observed in 15% of patients treated with mirtazapine, compared to 7% for placebo and 8% for amitriptyline. In these same studies, nonfasting triglyceride increases to ≥500 mg/dL were observed in 6% of patients treated with mirtazapine, compared to 3% for placebo and 3% for amitriptyline.
Transaminase Elevations
Clinically significant ALT (SGPT) elevations (≥3 times the upper limit of the normal range) were observed in 2.0% (8/424) of patients exposed to mirtazapine in a pool of short-term U.S. controlled trials, compared to 0.3% (1/328) of placebo patients and 2.0% (3/181) of amitriptyline patients. Most of these patients with ALT increases did not develop signs or symptoms associated with compromised liver function. While some patients were discontinued for the ALT increases, in other cases, the enzyme levels returned to normal despite continued mirtazapine treatment. Mirtazapine should be used with caution in patients with impaired hepatic function (see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION).
Activation of Mania/Hypomania
Mania/hypomania occurred in approximately 0.2% (3/1,299 patients) of mirtazapine treated patients in U.S. studies. Although the incidence of mania/hypomania was very low during treatment with mirtazapine, it should be used carefully in patients with a history of mania/hypomania.
Seizure
In premarketing clinical trials only one seizure was reported among the 2,796 U.S. and non-U.S. patients treated with mirtazapine. However, no controlled studies have been carried out in patients with a history of seizures. Therefore, care should be exercised when mirtazapine is used in these patients.
Use in Patients with Concomitant Illness
Clinical experience with mirtazapine in patients with concomitant systemic illness is limited. Accordingly, care is advisable in prescribing mirtazapine for patients with diseases or conditions that affect metabolism or hemodynamic responses.
Mirtazapine has not been systematically evaluated or used to any appreciable extent in patients with a recent history of myocardial infarction or other significant heart disease. Mirtazapine was associated with significant orthostatic hypotension in early clinical pharmacology trials with normal volunteers. Orthostatic hypotension was infrequently observed in clinical trials with depressed patients. Mirtazapine should be used with caution in patients with known cardiovascular or cerebrovascular disease that could be exacerbated by hypotension (history of myocardial infarction, angina, or ischemic stroke) and conditions that would predispose patients to hypotension (dehydration, hypovolemia, and treatment with antihypertensive medication).
Mirtazapine clearance is decreased in patients with moderate [glomerular filtration rate (GFR) = 11 to 39 mL/min/1.73 m2] and severe [GFR < 10 mL/min/1.73 m2] renal impairment, and also in patients with hepatic impairment. Caution is indicated in administering mirtazapine to such patients (see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION).
Information for patients
Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with mirtazapine and should counsel them in its appropriate use. A patient Medication Guide about “Antidepressant Medicines, Depression and other Serious Mental Illness, and Suicidal Thoughts or Actions” is available for mirtazapine. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. The complete text of the Medication Guide is reprinted at the end of this document.
Patients should be advised of the following issues and asked to alert their prescriber if these occur while taking mirtazapine.
Clinical Worsening and Suicide Risk
Patients, their families, and their caregivers should be encouraged to be alert to the emergence of anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, mania, other unusual changes in behavior, worsening of depression, and suicidal ideation, especially early during antidepressant treatment and when the dose is adjusted up or down. Families and caregivers of patients should be advised to look for the emergence of such symptoms on a day-to-day basis, since changes may be abrupt. Such symptoms should be reported to the patient's prescriber or health professional, especially if they are severe, abrupt in onset, or were not part of the patient's presenting symptoms. Symptoms such as these may be associated with an increased risk for suicidal thinking and behavior and indicate a need for very close monitoring and possibly changes in the medication.
Agranulocytosis
Patients who are to receive mirtazapine should be warned about the risk of developing agranulocytosis. Patients should be advised to contact their physician if they experience any indication of infection such as fever, chills, sore throat, mucous membrane ulceration or other possible signs of infection. Particular attention should be paid to any flu-like complaints or other symptoms that might suggest infection.
Interference with Cognitive and Motor Performance
Mirtazapine may impair judgement, thinking, and particularly, motor skills, because of its prominent sedative effect. The drowsiness associated with mirtazapine use may impair a patient’s ability to drive, use machines or perform tasks that require alertness. Thus, patients should be cautioned about engaging in hazardous activities until they are reasonably certain that mirtazapine therapy does not adversely affect their ability to engage in such activities.
Completing Course of Therapy
While patients may notice improvement with mirtazapine therapy in 1 to 4 weeks, they should be advised to continue therapy as directed.
Concomitant Medication
Patients should be advised to inform their physician if they are taking, or intend to take, any prescription or over-the-counter drugs since there is a potential for mirtazapine to interact with other drugs.
Alcohol
The impairment of cognitive and motor skills produced by mirtazapine has been shown to be additive with those produced by alcohol. Accordingly, patients should be advised to avoid alcohol while taking mirtazapine.
Pregnancy
Patients should be advised to notify their physician if they become pregnant or intend to become pregnant during mirtazapine therapy.
Nursing
Patients should be advised to notify their physician if they are breast-feeding an infant.
Laboratory tests
There are no routine laboratory tests recommended.
Drug Interactions
As with other drugs, the potential for interaction by a variety of mechanisms (e.g., pharmacodynamic, pharmacokinetic inhibition or enhancement, etc.) is a possibility (see CLINICAL PHARMACOLOGY).
Drugs Affecting Hepatic Metabolism
The metabolism and pharmacokinetics of mirtazapine may be affected by the induction or inhibition of drug-metabolizing enzymes.
Drugs that are Metabolized by and/or Inhibit Cytochrome P450 Enzymes
Many drugs are metabolized by and/or inhibit various cytochrome P450 enzymes, e.g., 2D6, 1A2, 3A4, etc. In vitro studies have shown that mirtazapine is a substrate for several of these enzymes, including 2D6, 1A2, and 3A4. While in vitro studies have shown that mirtazapine is not a potent inhibitor of any of these enzymes, an indication that mirtazapine is not likely to have a clinically significant inhibitory effect on the metabolism of other drugs that are substrates for these cytochrome P450 enzymes, the concomitant use of mirtazapine with most other drugs metabolized by these enzymes has not been formally studied. Consequently, it is not possible to make any definitive statements about the risks of coadministration of mirtazapine with such drugs.
Alcohol
Concomitant administration of alcohol (equivalent to 60 g) had a minimal effect on plasma levels of mirtazapine (15 mg) in 6 healthy male subjects. However, the impairment of cognitive and motor skills produced by mirtazapine were shown to be additive with those produced by alcohol. Accordingly, patients should be advised to avoid alcohol while taking mirtazapine.
Diazepam
Concomitant administration of diazepam (15 mg) had a minimal effect on plasma levels of mirtazapine (15 mg) in 12 healthy subjects. However, the impairment of motor skills produced by mirtazapine has been shown to be additive with those caused by diazepam. Accordingly, patients should be advised to avoid diazepam and other similar drugs while taking mirtazapine.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies were conducted with mirtazapine given in the diet of doses of 2, 20, and 200 mg/kg/day to mice and 2, 20, and 60 mg/kg/day to rats. The highest doses used are approximately 20 and 12 times the maximum recommended human dose (MRHD) of 45 mg/day on a mg/m2 basis in mice and rats, respectively. There was an increased incidence of hepatocellular adenoma and carcinoma in male mice at the high dose. In rats, there was an increase in hepatocellular adenoma in females at the mid and high doses and in hepatocellular tumors and thyroid follicular adenoma/cystadenoma and carcinoma in males at the high dose. The data suggest that the above effects could possibly be mediated by non-genotoxic mechanisms, the relevance of which to humans is not known.
The doses used in the mouse study may not have been high enough to fully characterize the carcinogenic potential of mirtazapine.
Mutagenesis
Mirtazapine was not mutagenic or clastogenic and did not induce general DNA damage as determined in several genotoxicity tests: Ames test, in vitro gene mutation assay in Chinese hamster V 79 cells, in vitro sister chromatid exchange assay in cultured rabbit lymphocytes, in vivo bone marrow micronucleus test in rats, and unscheduled DNA synthesis assay in HeLa cells.
Impairment of Fertility
In a fertility study in rats, mirtazapine was given at doses up to 100 mg/kg (20 times the maximum recommended human dose (MRHD) on a mg/m2 basis). Mating and conception were not affected by the drug, but estrous cycling was disrupted at doses that were 3 or more times the MRHD and pre-implantation losses occurred at 20 times the MRHD.
Pregnancy
Teratogenic effects - Pregnancy Category C
Reproduction studies in pregnant rats and rabbits at doses up to 100 mg/kg and 40 mg/kg, respectively (20 and 17 times the maximum recommended human dose (MRHD) on a mg/m2 basis, respectively), have revealed no evidence of teratogenic effects. However, in rats, there was an increase in post-implantation losses in dams treated with mirtazapine. There was an increase in pup deaths during the first 3 days of lactation and a decrease in pup birth weights. The cause of these deaths is not known. The effects occurred at doses that were 20 times the MRHD, but not at 3 times the MRHD, on a mg/m2 basis. There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing mothers
It is not known whether mirtazapine is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when mirtazapine is administered to nursing women.
Pediatric use
Safety and effectiveness in pediatric patients have not been established (see BOX WARNING and WARNINGS - Clinical Worsening and Suicide Risk). Two placebo-controlled trials in 258 pediatric patients with MDD have been conducted with mirtazapine, and the data were not sufficient to support a claim for use in pediatric patients. Anyone considering the use of mirtazapine in a child or adolescent must balance the potential risks with the clinical need.
Geriatric use
Approximately 190 elderly individuals (≥ 65 years of age) participated in clinical studies with mirtazapine. This drug is known to be substantially excreted by the kidney (75%), and the risk of decreased clearance of this drug is greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection. Sedating drugs may cause confusion and oversedation in the elderly. No unusual adverse age-related phenomena were identified in this group. Pharmacokinetic studies revealed a decreased clearance in the elderly. Caution is indicated in administering mirtazapine to elderly patients (see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION).
MIRTAZAPINE ADVERSE REACTIONS
Associated with Discontinuation of Treatment
Approximately 16 percent of the 453 patients who received mirtazapine in U.S. 6-week controlled clinical trials discontinued treatment due to an adverse experience, compared to 7 percent of the 361 placebo-treated patients in those studies. The most common events (≥1%) associated with discontinuation and considered to be drug related (i.e., those events associated with dropout at a rate at least twice that of placebo) included:
Common Adverse Events Associated with Discontinuation of Treatment in 6-Week U.S. Mirtazapine Trials | ||
Adverse Event | Percentage of Patients Discontinuing with Adverse Event | |
Mirtazapine (n=453) | Placebo (n=361) | |
| Somnolence | 10.4% | 2.2% |
| Nausea | 1.5% | 0% |
Commonly Observed Adverse Events in U.S. Controlled Clinical Trials
The most commonly observed adverse events associated with the use of mirtazapine (incidence of 5% or greater) and not observed at an equivalent incidence among placebo-treated patients (mirtazapine incidence at least twice that for placebo) were:
Common Treatment –Emergent Adverse Events Associated | ||
Adverse Event | Percentage of Patients Reporting Adverse Event | |
Mirtazapine (n=453) | Placebo (n=361) | |
| Somnolence | 54% | 18% |
Increased Appetite | 17% | 2% |
| Weight Gain | 12% | 2% |
| Dizziness | 7% | 3% |
Adverse Events Occurring at an Incidence of 1% or More Among Mirtazapine-Treated Patients
The table that follows enumerates adverse events that occurred at an incidence of 1% or more, and were more frequent than in the placebo group, among mirtazapine-treated patients who participated in short-term U.S. placebo-controlled trials in which patients were dosed in a range of 5 to 60 mg/day. This table shows the percentage of patients in each group who had at least one episode of an event at some time during their treatment. Reported adverse events were classified using a standard COSTART-based dictionary terminology.
The prescriber should be aware that these figures cannot be used to predict the incidence of side effects in the course of usual medical practice where patient characteristics and other factors differ from those which prevailed in the clinical trials. Similarly, the cited frequencies cannot be compared with figures obtained from other investigations involving different treatments, uses and investigators. The cited figures, however, do provide the prescribing physician with some basis for estimating the relative contribution of drug and non-drug factors to the side effect incidence rate in the population studied.
Body System/ Adverse Clinical Experience | Mirtazapine (n=453) | Placebo (n=361) |
| Body as a Whole | ||
| Asthenia | 8% | 5% |
| Flu Syndrome | 5% | 3% |
| Back Pain | 2% | 1% |
| Digestive System | ||
| Dry Mouth | 25% | 15% |
| Increased Appetite | 17% | 2% |
| Constipation | 13% | 7% |
| Metabolic and Nutritional Disorders | ||
| Weight Gain | 12% | 2% |
| Peripheral Edema | 2% | 1% |
| Edema | 1% | 0% |
| Musculoskeletal System | ||
| Myalgia | 2% | 1% |
| Nervous System | ||
| Somnolence | 54% | 18% |
| Dizziness | 7% | 3% |
| Abnormal Dreams | 4% | 1% |
| Thinking Abnormal | 3% | 1% |
| Tremor | 2% | 1% |
| Confusion | 2% | 0% |
| Respiratory System | ||
| Dyspnea | 1% | 0% |
| Urogenital System | ||
| Urinary Frequency | 2% | 1% |
1Events reported by at least 1% of patients treated with mirtazapine are included, except the following events which had an incidence on placebo ≥ mirtazapine: headache, infection, pain, chest pain, palpitation, tachycardia, postural hypotension, nausea, dyspepsia, diarrhea, flatulence, insomnia, nervousness, libido decreased, hypertonia, pharyngitis, rhinitis, sweating, amblyopia, tinnitus, taste perversion.
ECG Changes
The electrocardiograms for 338 patients who received mirtazapine and 261 patients who received placebo in 6-week, placebo-controlled trials were analyzed. Prolongation in QTc ≥500 msec was not observed among mirtazapine-treated patients; mean change in QTc was +1.6 msec for mirtazapine and -3.1 msec for placebo. Mirtazapine was associated with a mean increase in heart rate of 3.4 bpm, compared to 0.8 bpm for placebo. The clinical significance of these changes is unknown.
Other Adverse Events Observed During the Premarketing Evaluation of Mirtazapine
During its premarketing assessment, multiple doses of mirtazapine were administered to 2,796 patients in clinical studies. The conditions and duration of exposure to mirtazapine varied greatly, and included (in overlapping categories) open and double-blind studies, uncontrolled and controlled studies, inpatient and outpatient studies, fixed dose and titration studies. Untoward events associated with this exposure were recorded by clinical investigators using terminology of their own choosing. Consequently, it is not possible to provide a meaningful estimate of the proportion of individuals experiencing adverse events without first grouping similar types of untoward events into a smaller number of standardized event categories.
In the tabulations that follow, reported adverse events were classified using a standard COSTART-based dictionary terminology. The frequencies presented, therefore, represent the proportion of the 2,796 patients exposed to multiple doses of mirtazapine who experienced an event of the type cited on at least one occasion while receiving mirtazapine. All reported events are included except those already listed in the previous table, those adverse experiences subsumed under COSTART terms that are either overly general or excessively specific so as to be uninformative, and those events for which a drug cause was very remote.
It is important to emphasize that, although the events reported occurred during treatment with mirtazapine, they were not necessarily caused by it.
Events are further categorized by body system and listed in order of decreasing frequency according to the following definitions: frequent adverse events are those occurring on one or more occasions in at least 1/100 patients; infrequent adverse events are those occurring in 1/100 to 1/1000 patients; rare events are those occurring in fewer than 1/1000 patients. Only those events not already listed in the previous table appear in this listing. Events of major clinical importance are also described in the WARNINGS and PRECAUTIONS sections.
Body as a Whole: frequent: malaise, abdominal pain, abdominal syndrome acute; infrequent: chills, fever, face edema, ulcer, photosensitivity reaction, neck rigidity, neck pain, abdomen enlarged; rare: cellulitis, chest pain substernal.
Cardiovascular System: frequent: hypertension, vasodilatation; infrequent: angina pectoris, myocardial infarction, bradycardia, ventricular extrasystoles, syncope, migraine, hypotension; rare: atrial arrhythmia, bigeminy, vascular headache, pulmonary embolus, cerebral ischemia, cardiomegaly, phlebitis, left heart failure.
Digestive System:frequent: vomiting, anorexia; infrequent: eructation, glossitis, cholecystitis, nausea and vomiting, gum hemorrhage, stomatitis, colitis, liver function tests abnormal; rare: tongue discoloration, ulcerative stomatitis, salivary gland enlargement, increased salivation, intestinal obstruction, pancreatitis, aphthous stomatitis, cirrhosis of liver, gastritis, gastroenteritis, oral moniliasis, tongue edema.
Endocrine System: rare: goiter, hypothyroidism.
Hemic and Lymphatic System: rare: lymphadenopathy, leukopenia, petechia, anemia, thrombocytopenia, lymphocytosis, pancytopenia.
Metabolic and Nutritional Disorders: frequent: thirst; infrequent: dehydration, weight loss; rare: gout, SGOT increased, healing abnormal, acid phosphatase increased, SGPT increased, diabetes mellitus.
Musculoskeletal System: frequent: myasthenia, arthralgia; infrequent:arthritis, tenosynovitis; rare: pathologic fracture, osteoporosis fracture, bone pain, myositis, tendon rupture, arthrosis, bursitis.
Nervous System: frequent: hypesthesia, apathy, depression, hypokinesia, vertigo, twitching, agitation, anxiety, amnesia, hyperkinesia, paresthesia; infrequent: ataxia, delirium, delusions, depersonalization, dyskinesia, extrapyramidal syndrome, libido increased, coordination abnormal, dysarthria, hallucinations, manic reaction, neurosis, dystonia, hostility, reflexes increased, emotional lability, euphoria, paranoid reaction; rare: aphasia, nystagmus, akathisia, stupor, dementia, diplopia, drug dependence, paralysis, grand mal convulsion, hypotonia, myoclonus, psychotic depression, withdrawal syndrome.
Respiratory System: frequent: cough increased, sinusitis; infrequent: epistaxis, bronchitis, asthma, pneumonia; rare: asphyxia, laryngitis, pneumothorax, hiccup.
Skin and Appendages: frequent: pruritus, rash; infrequent: acne, exfoliative dermatitis, dry skin, herpes simplex, alopecia; rare: urticaria, herpes zoster, skin hypertrophy, seborrhea, skin ulcer.
Special Senses: infrequent: eye pain, abnormality of accommodation, conjunctivitis, deafness, keratoconjunctivitis, lacrimation disorder, glaucoma, hyperacusis, ear pain; rare: blepharitis, partial transitory deafness, otitis media, taste loss, parosmia.
Urogenital System: frequent: urinary tract infection; infrequent: kidney calculus, cystitis, dysuria, urinary incontinence, urinary retention, vaginitis, hematuria, breast pain, amenorrhea, dysmenorrhea, leukorrhea, impotence; rare: polyuria, urethritis, metrorrhagia, menorrhagia, abnormal ejaculation, breast engorgement, breast enlargement, urinary urgency.
Other Adverse Events Observed During Postmarketing Evaluation of Mirtazapine
Adverse events reported since market introduction, which were temporally (but not necessarily causally) related to mirtazapine therapy, include four cases of the ventricular arrhythmia torsades de pointes. In three of the four cases, however, concomitant drugs were implicated. All patients recovered.
DRUG ABUSE AND DEPENDENCE
Controlled Substance Class
Mirtazapine tablets are not a controlled substance.
Physical and Psychological Dependence
Mirtazapine has not been systematically studied in animals or humans for its potential for abuse, tolerance or physical dependence. While the clinical trials did not reveal any tendency for any drug-seeking behavior, these observations were not systematic and it is not possible to predict on the basis of this limited experience the extent to which a CNS-active drug will be misused, diverted and/or abused once marketed. Consequently, patients should be evaluated carefully for history of drug abuse, and such patients should be observed closely for signs of mirtazapine misuse or abuse (e.g., development of tolerance, incrementations of dose, drug-seeking behavior).
OVERDOSAGE
Human Experience
There is very limited experience with mirtazapine overdose. In premarketing clinical studies, there were eight reports of mirtazapine overdose alone or in combination with other pharmacological agents. The only drug overdose death reported while taking mirtazapine was in combination with amitriptyline and chlorprothixene in a non-U.S. clinical study. Based on plasma levels, the mirtazapine dose taken was 30 to 45 mg, while plasma levels of amitriptyline and chlorprothixene were found to be at toxic levels. All other premarketing overdose cases resulted in full recovery. Signs and symptoms reported in association with overdose included disorientation, drowsiness, impaired memory, and tachycardia. There were no reports of ECG abnormalities, coma or convulsions following overdose with mirtazapine alone.
Overdose Management
Treatment should consist of those general measures employed in the management of overdose with any drug effective in the treatment of major depressive disorder. Ensure an adequate airway, oxygenation, and ventilation. Monitor cardiac rhythm and vital signs. General supportive and symptomatic measures are also recommended. Induction of emesis is not recommended. Gastric lavage with a large-bore orogastric tube with appropriate airway protection, if needed, may be indicated if performed soon after ingestion, or in symptomatic patients.
Activated charcoal should be administered. There is no experience with the use of forced diuresis, dialysis, hemoperfusion or exchange transfusion in the treatment of mirtazapine overdosage. No specific antidotes for mirtazapine are known.
In managing overdosage, consider the possibility of multiple-drug involvement. The physician should consider contacting a poison control center for additional information on the treatment of any overdose. Telephone numbers for certified poison control centers are listed in the Physicians’ Desk Reference (PDR).
MIRTAZAPINE DOSAGE AND ADMINISTRATION
Initial Treatment
The recommended starting dose for mirtazapine tablets is 15 mg/day, administered in a single dose, preferably in the evening prior to sleep. In the controlled clinical trials establishing the efficacy of mirtazapine in the treatment of major depressive disorder, the effective dose range was generally 15 to 45 mg/day. While the relationship between dose and satisfactory response in the treatment of major depressive disorder for mirtazapine has not been adequately explored, patients not responding to the initial 15 mg dose may benefit from dose increases up to a maximum of 45 mg/day. Mirtazapine has an elimination half-life of approximately 20 to 40 hours; therefore, dose changes should not be made at intervals of less than one to two weeks in order to allow sufficient time for evaluation of the therapeutic response to a given dose.
Elderly and Patients with Renal or Hepatic Impairment
The clearance of mirtazapine is reduced in elderly patients and in patients with moderate to severe renal or hepatic impairment. Consequently, the prescriber should be aware that plasma mirtazapine levels may be increased in these patient groups, compared to levels observed in younger adults without renal or hepatic impairment (see PRECAUTIONS and CLINICAL PHARMACOLOGY).
Maintenance/Extended Treatment
It is generally agreed that acute episodes of depression require several months or longer of sustained pharmacological therapy beyond response to the acute episode. Systematic evaluation of mirtazapine has demonstrated that its efficacy in major depressive disorder is maintained for periods of up to 40 weeks following 8 to 12 weeks of initial treatment at a dose of 15 to 45 mg/day (see CLINICAL PHARMACOLOGY). Based on these limited data, it is unknown whether or not the dose of mirtazapine needed for maintenance treatment is identical to the dose needed to achieve an initial response. Patients should be periodically reassessed to determine the need for maintenance treatment and the appropriate dose for such treatment.
Switching Patients To or From a Monoamine Oxidase Inhibitor
At least 14 days should elapse between discontinuation of an MAOI and initiation of therapy with mirtazapine. In addition, at least 14 days should be allowed after stopping mirtazapine before starting an MAOI.
HOW SUPPLIED
Mirtazapine Tablets are supplied as:
15 mg Tablets -normal convex, scored, yellow, film-coated, debossed “MR|15” on one side and “G” on the other.
Bottles of 30 NDC57315-078-01
Bottles of 100 NDC57315-078-02
Bottles of 1000 NDC57315-078-03
Unit Dose, Box of 100 NDC57315-078-04*
30 mg Tablets - normal convex, scored, buff, film-coated, debossed “MR|30” on one side
and “G” on the other.
Bottles of 30 NDC57315-079-01
Bottles of 100 NDC57315-079-02
Bottles of 1000 NDC57315-079-03
45 mg Tablets - normal convex, white, film-coated, debossed “MR 45” on one side and “G” on the other.
Bottles of 30 NDC57315-080-01
Bottles of 1000 NDC57315-080-02
*Unit dose packs are provided as a blisterpack with 10 strips, each of which contains 10 tablets.
Storage
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Dispense in a tight, light resistant container as described in the USP.
MANUFACTURED BY:
ALPHAPHARM PTY LTD
15 Garnet Street
Carole Park QLD 4300
Australia
1159/7 Revised May 2007
Mirtazapine Tablets
Rx only
Antidepressant Medicines, Depression and other Serious Mental Illnesses,and Suicidal Thoughts or Actions
Read the Medication Guide that comes with you or your family member’s antidepressant medicine.
This Medication Guide is only about the risk of suicidal thoughts and actions with antidepressant medicines. Talk to your, or your family member’s, healthcare provider about:
• all risks and benefits of treatment with antidepressant medicines
• all treatment choices for depression or other serious mental illness
What is the most important information I should know about antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions?
- Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults when the medicine is first started.
- Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions. These include people who have (or have a family history of) bipolar illness (also called manic-depressive illness) or suicidal thoughts or actions.
- How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
- Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is first started or when the dose is changed.
- Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
|
|
What else do I need to know about antidepressant medicines?
- Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms.
- Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
- Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
- Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
- Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child’s healthcare provider for more information.
This Medication Guide has been approved by the U.S. Food and Drug Administration for all antidepressants
MANUFACTURED BY:
ALPHAPHARM PTY LTD
15 Garnet Street
Carole Park QLD 4300
Australia
1159/7 Revised May 2007
MirtazapineMirtazapine TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
MirtazapineMirtazapine TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
MirtazapineMirtazapine TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||