Mirtazapine
Lake Erie Medical DBA Quality Care Products LLC
FULL PRESCRIBING INFORMATION: CONTENTS*
- Suicidality and Antidepressant Drugs
- MIRTAZAPINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- MIRTAZAPINE INDICATIONS AND USAGE
- MIRTAZAPINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- MIRTAZAPINE ADVERSE REACTIONS
- Associated with Discontinuation of Treatment
- Commonly Observed Adverse Events in US Controlled Clinical Trials
- Adverse Events Occurring at an Incidence of 1% or More Among Mirtazapine-Treated Patients
- ECG Changes
- Other Adverse Events Observed During the Premarketing Evaluation of Mirtazapine
- Other Adverse Events Observed During Postmarketing Evaluation of Mirtazapine
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- Medication Guide
- Image of Label
FULL PRESCRIBING INFORMATION
Suicidality and Antidepressant Drugs
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of mirtazapine tablets or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Mirtazapine is not approved for use in pediatric patients. (See WARNINGS: Clinical Worsening and Suicide Risk, PRECAUTIONS: Information for Patients, and PRECAUTIONS: Pediatric Use)
MIRTAZAPINE DESCRIPTION
Mirtazapine tablets, USP are an orally administered drug. Mirtazapine has a tetracyclic chemical structure and belongs to the piperazino-azepine group of compounds. It is designated 1,2,3,4,10,14b-hexahydro-2-methylpyrazino[2,1-a] pyrido [2,3-c] benzazepine and has the empirical formula of C17H19N3. Its molecular weight is 265.36. The structural formula is the following and it is the racemic mixture:
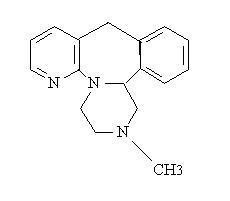
Mirtazapine USP is a white to creamy white crystalline powder which is slightly soluble in water.
Mirtazapine tablets, USP are supplied for oral administration as scored film-coated tablets containing 15 or 30 mg of mirtazapine USP, and unscored film-coated tablets containing 7.5 or 45 mg of mirtazapine USP. Each tablet also contains corn starch, hydroxypropyl cellulose, magnesium stearate, colloidal silicon dioxide, lactose monohydrate, hypromellose and titanium dioxide. In addition, the 15 mg contains iron oxide yellow and 30 mg contains iron oxide red, iron oxide black and iron oxide yellow.
CLINICAL PHARMACOLOGY
Pharmacodynamics
2
2 31A1B
1
1
Pharmacokinetics
In vitro
Special Populations
Geriatric
PRECAUTIONSDOSAGE AND ADMINISTRATION
Pediatrics
PRECAUTIONS
Gender
Pharmacokinetics
Race
Renal Insufficiency
22PRECAUTIONSDOSAGE AND ADMINISTRATION
Hepatic Insufficiency
PRECAUTIONSDOSAGE AND ADMINISTRATION
Clinical Trials Showing Effectiveness
MIRTAZAPINE INDICATIONS AND USAGE
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY
MIRTAZAPINE CONTRAINDICATIONS
Hypersensitivity
Monoamine Oxidase Inhibitors
WARNINGSPRECAUTIONS: Drug InteractionsDOSAGE AND ADMINISTRATION
WARNINGS
Clinical Worsening and Suicide Risk
Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment. Pooled analyses of short-term placebo-controlled trials of antidepressant drugs (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18 to 24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older.
| Table 1 | |
|---|---|
| Age Range |
Drug-Placebo Difference in Number of Cases of Suicidality per 1000 Patients Treated |
| |
Increases Compared to Placebo |
| <18 |
14 additional cases |
| 18-24 |
5 additional cases |
| |
Decreases Compared to Placebo |
| 25-64 |
1 fewer case |
| ≥65 |
6 fewer cases |
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patient's presenting symptoms.
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to health care providers. Such monitoring should include daily observation by families and caregivers. Prescriptions for Mirtazapine tablets should be written for the smallest quantity of tablets consistent with good patient management, in order to reduce the risk of overdose.
Screening Patients for Bipolar Disorder:
Agranulocytosis
In premarketing clinical trials, 2 (1 with Sjögren’s Syndrome) out of 2,796 patients treated with mirtazapine tablets developed agranulocytosis [absolute neutrophil count (ANC) < 500/mm3 with associated signs and symptoms, e.g., fever, infection, etc.] and a third patient developed severe neutropenia (ANC < 500/mm3 without any associated symptoms). For these 3 patients, onset of severe neutropenia was detected on days 61, 9, and 14 of treatment, respectively. All 3 patients recovered after mirtazapine was stopped. These 3 cases yield a crude incidence of severe neutropenia (with or without associated infection) of approximately 1.1 per thousand patients exposed, with a very wide 95% confidence interval i.e., 2.2 cases per 10,000 to 3.1 cases per 1,000. If a patient develops a sore throat, fever, stomatitis or other signs of infection, along with a low WBC count, treatment with mirtazapine should be discontinued and the patient should be closely monitored.
MAO Inhibitors
In patients receiving other drugs for major depressive disorder in combination with a monoamine oxidase inhibitor (MAOI) and in patients who have recently discontinued a drug for major depressive disorder and then are started on an MAOI, there have been reports of serious and sometimes fatal, reactions, including nausea, vomiting, flushing, dizziness, tremor, myoclonus, rigidity, diaphoresis, hyperthermia, autonomic instability with rapid fluctuations of vital signs, seizures, and mental status changes ranging from agitation to coma. Although there are no human data pertinent to such an interaction with mirtazapine tablets, it is recommended that mirtazapine not be used in combination with an MAOI, or within 14 days of initiating or discontinuing therapy with an MAOI.
Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-like Reactions
CONTRAINDICATIONS
If concomitant treatment of mirtazapine tablets with a 5-hydroxytryptamine receptor agonist (triptan) is clinically warranted, careful observation of the patient is advised, particularly during treatment initiation and dose increases.
The concomitant use of mirtazapine tablets with serotonin precursors (such as tryptophan) is not recommended.
Treatment with mirtazapine tablets and any concomitant serotonergic or antidopaminergic agents, including antipsychotics, should be discontinued immediately if the above events occur and supportive symptomatic treatment should be initiated.
PRECAUTIONS
General
Discontinuation Symptoms
Akathisia/Psychomotor Restlessness
Hyponatremia
Somnolence
Information for Patients
Dizziness
Increased Appetite/Weight Gain
PRECAUTIONS: Pediatric Use
Cholesterol/Triglycerides
Transaminase Elevations
CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATION
Activation of Mania/Hypomania
Seizure
Use in Patients with Concomitant Illness
22CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATION
Information for Patients
Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with Mirtazapine tablets and should counsel them in its appropriate use. A patient Medication Guide about “Antidepressant Medicines, Depression and other Serious Mental Illnesses, and Suicidal Thoughts or Actions” is available for mirtazapine. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. The complete text of the Medication Guide is reprinted at the end of this document.
Patients should be advised of the following issues and asked to alert their prescriber if these occur while taking mirtazapine.
Clinical Worsening and Suicide Risk
Agranulocytosis
Interference with Cognitive and Motor Performance
Completing Course of Therapy
Concomitant Medication
Alcohol
Pregnancy
Nursing
Laboratory Tests
DRUG INTERACTIONS
CLINICAL PHARMACOLOGY
Monoamine Oxidase Inhibitors
CONTRAINDICATIONSWARNINGSDOSAGE AND ADMINISTRATION
Serotonergic Drugs
CONTRAINDICATIONSWARNINGS
Drugs Affecting Hepatic Metabolism
Drugs that are Metabolized by and/or Inhibit Cytochrome P450 Enzymes
CYP Enzyme Inducers (these studies used both drugs at steady state)
Phenytoin
Carbamazepine
CYP Enzyme Inhibitors
Cimetidine
Ketoconazole
Paroxetine
in vivo
Other Drug-Drug Interactions
Amitriptyline
Warfarin
Lithium
Risperidone
in vivo
Alcohol
Diazepam
Carcinogenesis, Mutagenesis, Impairment of Fertility
2
in vitro in vitro in vivo
2
Pregnancy
Teratogenic Effects – Pregnancy Category C
22
Nursing Mothers
Because some mirtazapine may be excreted into breast milk, caution should be exercised when mirtazapine tablets are administered to nursing women.
Pediatric Use
Safety and effectiveness in the pediatric population have not been established (see BOX WARNING and WARNINGS—Clinical Worsening and Suicide Risk). Two placebo-controlled trials in 258 pediatric patients with MDD have been conducted with mirtazapine tablets, and the data were not sufficient to support a claim for use in pediatric patients. Anyone considering the use of mirtazapine tablets in a child or adolescent must balance the potential risks with the clinical need.
In an 8-week-long pediatric clinical trial of doses between 15 to 45 mg/day, 49% of mirtazapine-treated patients had a weight gain of at least 7%, compared to 5.7% of placebo-treated patients. The mean increase in weight was 4 kg (2 kg SD) for mirtazapine-treated patients versus 1 kg (2 kg SD) for placebo-treated patients (see PRECAUTIONS: Increased Appetite/Weight Gain).
Geriatric Use
CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATION
MIRTAZAPINE ADVERSE REACTIONS
Associated with Discontinuation of Treatment
Table 2: Common Adverse Events Associated with Discontinuation of Treatment in 6-Week US
Mirtazapine Trials
| Adverse Event |
Percentage of Patients Discontinuing with Adverse Event |
|
| Mirtazapine (n=453) |
Placebo (n=361) |
|
| Somnolence |
10.4% |
2.2% |
| Nausea |
1.5% |
0% |
Commonly Observed Adverse Events in US Controlled Clinical Trials
Table 3: Common Treatment –Emergent Adverse Events Associated with the Use of Mirtazapine
in 6-Week US Trials
| Adverse Event |
Percentage of Patients Reporting Adverse Event |
|
| Mirtazapine (n=453) |
Placebo (n=361) |
|
| Somnolence |
54% |
18% |
| Increased Appetite |
17% |
2% |
| Weight Gain |
12% |
2% |
| Dizziness |
7% |
3% |
Adverse Events Occurring at an Incidence of 1% or More Among Mirtazapine-Treated Patients
Table 4 enumerates adverse events that occurred at an incidence of 1% or more, and were more frequent than in the placebo group, among mirtazapine tablets-treated patients who participated in short-term US placebo-controlled trials in which patients were dosed in a range of 5 to 60 mg/day. This table shows the percentage of patients in each group who had at least 1 episode of an event at some time during their treatment. Reported adverse events were classified using a standard COSTART-based dictionary terminology.
Table 4: Incidence of Adverse Clinical Experiences1 (≥1%) In Short-Term U.S. Controlled Studies
| Body System Adverse Clinical Experience | Mirtazapine (n=453) |
Placebo (n=361) |
|---|---|---|
|
1Events reported by at least 1% of patients treated with mirtazapine are included, except the following events which had an incidence on placebo grater than or equal to mirtazapine: headache, infection, pain, chest pain, palpitation, tachycardia, postural hypotension, nausea, dyspepsia, diarrhea, flatulence, insomnia, nervousness, libido decreased, hypertonia, pharyngitis, rhinitis, sweating, amblyopia, tinnitus, taste perversion |
||
|
Body as a Whole
|
||
| Asthenia |
8% |
5% |
| Flu Syndrome |
5% |
3% |
| Back Pain |
2% |
1% |
|
Digestive System
|
|
|
| Dry Mouth |
25% |
15% |
| Increased Appetite |
17% |
2% |
| Constipation |
13% |
7% |
|
Metabolic and Nutritional Disorders
|
||
| Weight Gain |
12% |
2% |
| Peripheral Edema |
2% |
1% |
| Edema |
1% |
0% |
|
Musculoskeletal System
|
||
| Myalgia |
2% |
1% |
|
Nervous System
|
||
| Somnolence |
54% |
18% |
| Dizziness |
7% |
3% |
| Abnormal Dreams |
4% |
1% |
| Thinking Abnormal |
3% |
1% |
| Tremor |
2% |
1% |
| Confusion |
2% |
0% |
|
Respiratory System
|
||
| Dyspnea |
1% |
0% |
|
Urogenital System
|
||
| Urinary Frequency |
2% |
1% |
ECG Changes
Other Adverse Events Observed During the Premarketing Evaluation of Mirtazapine
WARNINGSPRECAUTIONS
Body as a Wholefrequent:infrequent: rare:
Cardiovascular Systemfrequent: infrequent:rare:
Digestive Systemfrequent: infrequent: rare:
Endocrine Systemrare:
Hemic and Lymphatic System rare:
Metabolic and Nutritional Disordersfrequent: infrequent: rare:
Musculoskeletal System:frequent: infrequent: rare:
Nervous Systemfrequent: infrequent: rare:
Respiratory Systemfrequent: infrequent: rare:
Skin and Appendagesfrequent: infrequent: rare:
Special Sensesinfrequent: rare:
Urogenital Systemfrequent:infrequent: rare:
Other Adverse Events Observed During Postmarketing Evaluation of Mirtazapine
DRUG ABUSE AND DEPENDENCE
Controlled Substance Class
Physical and Psychological Dependence
OVERDOSAGE
Human Experience
Overdose Management
Physicians’ Desk Reference (
DOSAGE AND ADMINISTRATION
Initial Treatment
Elderly and Patients with Renal or Hepatic Impairment
PRECAUTIONSCLINICAL PHARMACOLOGY
Maintenance/Extended Treatment
CLINICAL PHARMACOLOGY
Switching Patients To or From a Monoamine Oxidase Inhibitor
Discontinuation of Mirtazapine Tablets treatment
PRECAUTIONSADVERSE REACTIONS
HOW SUPPLIED
7.5 mg Tablets
15 mg Tablets
30 mg Tablets
45 mg Tablets
Storage
Store at
Aurolife Pharma LLC
Aurobindo Pharma USA, Inc.
Medication Guide
Mirtazapine Tablets, USP
What is the most important information I should know about mirtazapine tablets?
Mirtazapine tablets and other antidepressant medicines, may cause serious side effects, including:
1. Suicidal thoughts or actions:
- Mirtazapine tablets and other antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, or young adults within the first few months of treatment or when the dose is changed.
- Depression or other serious mental illnesses are the most important causes of suicidal thoughts or actions.
- Watch for these changes and call your healthcare provider right away if you notice:
-
New or sudden changes in mood, behavior, actions, thoughts, or feelings, especially if severe. -
Pay particular attention to such changes when mirtazapine tablets are started or when the dose is changed.
-
Call your healthcare provider right away if you have any of the following symptoms, or call 911 if an emergency, especially if they are new, worse, or worry you:
- attempts to commit suicide
- acting on dangerous impulses
- acting aggressive or violent
- thoughts about suicide or dying
- new or worse depression
- new or worse anxiety or panic attacks
- feeling agitated, restless, angry or irritable
- trouble sleeping
- an increase in activity or talking more than what is normal for you
- other unusual changes in behavior or mood
Call your healthcare provider right away if you have any of the following symptoms, or call 911 if an emergency. Mirtazapine tablets may be associated with these serious side effects:
2. Manic episodes:
- greatly increased energy
- severe trouble sleeping
- racing thoughts
- reckless behavior
- unusually grand ideas
- excessive happiness or irritability
- talking more or faster than usual
3. Decreased White Blood Cells
4. Serotonin Syndrome or Neuroleptic Malignant Syndrome-like reactions. This condition can be life-threatening and may include:
- agitation, hallucinations, coma or other changes in mental status
- coordination problems or muscle twitching (overactive reflexes)
- racing heartbeat, high or low blood pressure
- sweating or fever
- nausea, vomiting, or diarrhea
- muscle rigidity
5. Seizures
6. Low salt (sodium) levels in the blood.
- headache
- weakness or feeling unsteady
- confusion, problems concentrating or thinking or memory problems
7. Sleepiness. It is best to take mirtazapine tablets close to bedtime.
8. Severe skin reactions:
- severe rash with skin swelling (including on the palms of the hands and soles of the feet)
- painful reddening of the skin and/or blisters/ulcers on the body or in the mouth
9. Severe allergic reactions: trouble breathing, swelling of the face, tongue, eyes or mouth
- rash, itchy welts (hives) or blisters, alone or with fever or joint pain
10. Increases in appetite or weight. Children and adolescents should have height and weight monitored during treatment.
11. Increased cholesterol and triglyceride levels in your blood
Do not stop mirtazapine tablets without first talking to your healthcare provider . Stopping mirtazapine tablets too quickly may cause potentially serious symptoms including:
- dizziness
- abnormal dreams
- agitation
- anxiety
- fatigue
- confusion
- headache
- shaking
- tingling sensation
- nausea, vomiting
- sweating
What are mirtazapine tablets?
Mirtazapine tablets are a prescription medicine used to treat depression. It is important to talk with your healthcare provider about the risks of treating depression and also the risks of not treating it. You should discuss all treatment choices with your healthcare provider.
Talk to your healthcare provider if you do not think that your condition is getting better with mirtazapine tablets treatment.
Who should not take mirtazapine tablets?
Do not take mirtazapine tablets if you:
- are allergic to mirtazapine or any of the ingredients in mirtazapine tablets. See the end of this Medication Guide for a complete list of ingredients in mirtazapine tablets.
- take a Monoamine Oxidase Inhibitor (MAOI). Ask your healthcare provider or pharmacist if you are not sure if you take an MAOI, including the antibiotic linezolid.
- it is recommended that mirtazapine tablets not be used in combination with an MAOI within 14 days of initiating or discontinuing therapy with an MAOI.
People who take mirtazapine tablets close in time to an MAOI may have serious or even life-threatening side effects. Get medical help right away if you have any of these symptoms:
- high fever
- uncontrolled muscle spasms
- stiff muscles
- rapid changes in heart rate or blood pressure
- confusion
- loss of consciousness (pass out)
What should I tell my healthcare provider before taking mirtazapine tablets? Ask if you are not sure.
Before starting mirtazapine tablets, tell your healthcare provider if you:
- Are taking certain drugs such as:
- Triptans used to treat migraine headache
- Medicines used to treat mood, anxiety, psychotic or thought disorders, including tricyclics, lithium, SSRIs, SNRIs, or antipsychotics
- Tramadol used to treat pain
- Over-the-counter supplements such as tryptophan or St. John's Wort
- Phenytoin, carbamazepine, or rifampicin (these drugs can decrease your blood level of mirtazapine)
- Cimetidine or ketoconazole (these drugs can increase your blood level of mirtazapine)
- Have or had:
- liver problems
- kidney problems
- heart problems
- seizures or convulsions
- bipolar disorder or mania
- a tendency to get dizzy or faint
- are pregnant or plan to become pregnant. It is not known if mirtazapine tablets will harm your unborn baby. Talk to your healthcare provider about the benefits and risks of treating depression during pregnancy
- are breastfeeding or plan to breastfeed. Some mirtazapine may pass into your breast milk. Talk to your healthcare provider about the best way to feed your baby while taking mirtazapine tablets
Tell your healthcare provider about all the medicines that you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Mirtazapine tablets and some medicines may interact with each other, may not work as well, or may cause serious side effects.
| If you take mirtazapine tablets, you should not take any other medicines that contain mirtazapine including mirtazapine orally disintegrating tablets. |
How should I take mirtazapine tablets?
- Take mirtazapine tablets exactly as prescribed. Your healthcare provider may need to change the dose of mirtazapine tablets until it is the right dose for you.
- Take mirtazapine tablets at the same time each day, preferably in the evening at bedtime.
- Swallow mirtazapine tablets as directed.
- It is common for antidepressant medicines such as mirtazapine tablets to take up to a few weeks before you start to feel better. Do not stop taking mirtazapine tablets if you do not feel results right away.
- Do not stop taking or change the dose of mirtazapine tablets without first talking to your doctor, even if you feel better.
- Mirtazapine tablets may be taken with or without food.
- If you miss a dose of mirtazapine take the missed dose as soon as you remember. If it is almost time for the next dose, skip the missed dose and take your next dose at the regular time. Do not take two doses of mirtazapine tablets at the same time.
- If you take too much mirtazapine, call your healthcare provider or poison control center right away, or get emergency treatment.
What should I avoid while taking mirtazapine tablets?
- Mirtazapine tablets can cause sleepiness or may affect your ability to make decisions, think clearly, or react quickly. You should not drive, operate heavy machinery, or do other dangerous activities until you know how mirtazapine tablets affect you.
- Avoid drinking alcohol or taking diazepam (a medicine used for anxiety, insomnia and seizures, for example) or similar medicines while taking mirtazapine tablets. If you are uncertain about whether certain medication can be taken with mirtazapine tablets, please discuss with your doctor.
What are the possible side effects of mirtazapine tablets?
Mirtazapine tablets may cause serious side effects, including all of those described in the section entitled "What is the most important information I should know about mirtazapine tablets?"
Common possible side effects in people who take mirtazapine tablets include:
- sleepiness
- increased appetite, weight gain
- dry mouth
- constipation
- dizziness
- abnormal dreams
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of mirtazapine tablets. For more information, ask your healthcare provider or pharmacist.
CALL YOUR DOCTOR FOR MEDICAL ADVICE ABOUT SIDE EFFECTS. YOU MAY REPORT SIDE EFFECTS TO THE FDA AT 1-800-FDA-1088.
How should I store mirtazapine tablets?
- Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
- Protect from light and moisture.
Keep mirtazapine tablets and all medicines out of the reach of children.
General information about mirtazapine tablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use mirtazapine tablets for a condition for which it was not prescribed. Do not give mirtazapine tablets to other people, even if they have the same condition. They may harm them.
This Medication Guide summarizes the most important information about mirtazapine tablets. If you would like more information, talk with your healthcare provider. You may ask your healthcare provider or pharmacist for information about mirtazapine tablets that is written for healthcare professionals.
For more information about mirtazapine tablets, call 1-866-850-2876.
What are the ingredients in mirtazapine tablets?
Active ingredient: mirtazapine
Inactive ingredients: corn starch, hydroxypropyl cellulose, magnesium stearate, colloidal silicon dioxide, lactose monohydrate, hypromellose, and titanium dioxide. In addition, the 15 mg contains iron oxide yellow and 30 mg contains iron oxide red, iron oxide black, and iron oxide yellow.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Aurolife Pharma LLC
Dayton, NJ 08810
Manufactured for:
Aurobindo Pharma USA, Inc.
Dayton, NJ 08810
Image of Label

MirtazapineMirtazapine TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||