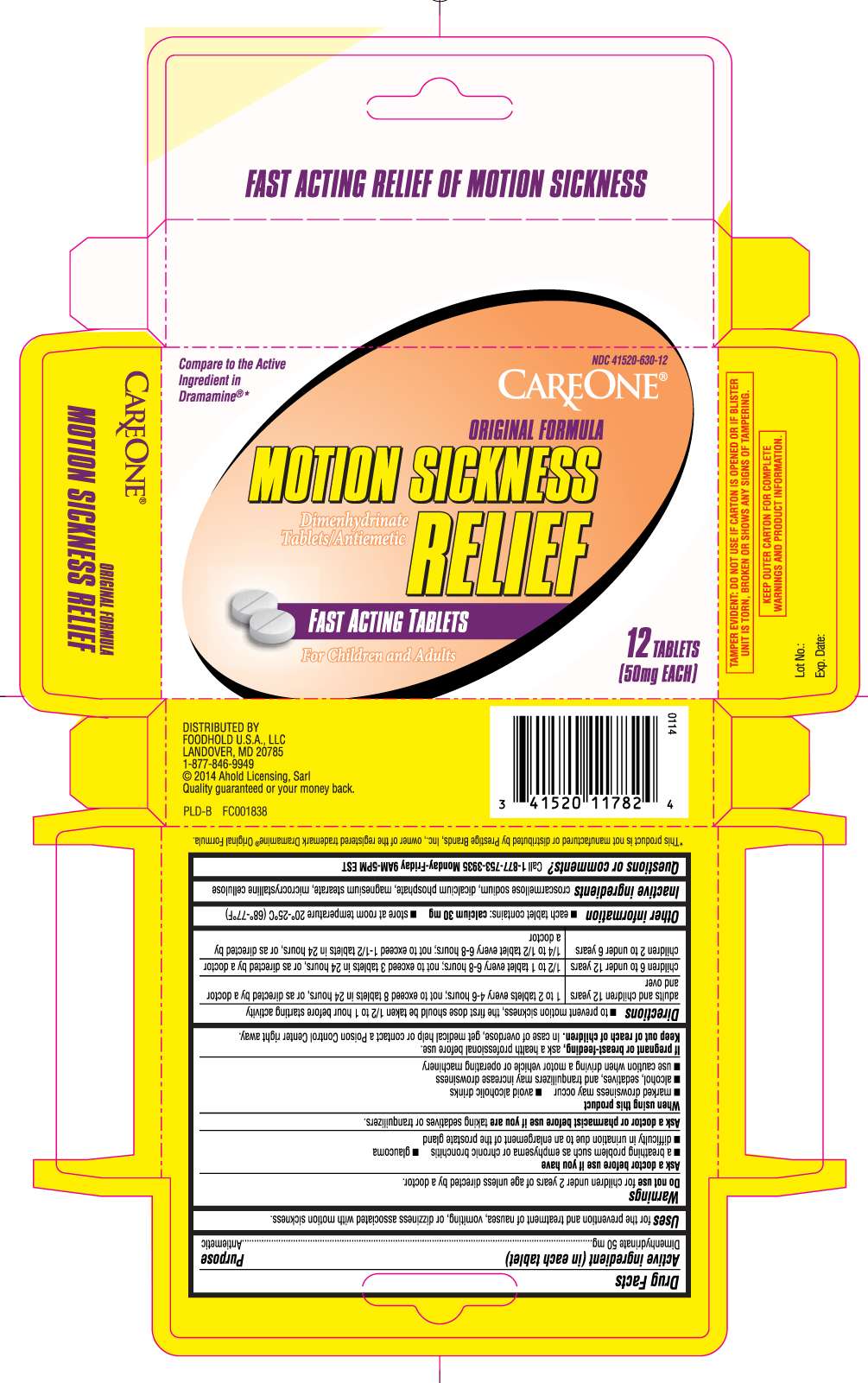
Motion Sickness Relief
Care One (American Sales Company)
P and L Development of New York Corporation
Dimenhydrinate 50 mg
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Drug Facts
Dimenhydrinate 50 mg
Antiemetic
for prevention and treatment of nausea, vomiting, or dizziness associated with motion sickness.
for children under 2 years of age, unless directed by a doctor
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
taking sedatives or tranquilizers.
- marked drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives and tranquilizers may increase drowsiness
- use caution when driving a motor vehicle or operating machinery
ask a health care professional before use.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- to prevent motion sickness, the first dose should be taken ½ to 1 hour before starting activity
| adults and children 12 years and over | 1 to 2 tablets every 4- 6 hours; not to exceed 8 tablets in 24 hours or as directed by a doctor |
| children 6 to under 12 years | take 1/2 to 1 tablet every 6-8 hours; not to exceed 3 tablets in 24 hours or as directed by a doctor |
| children 2 to under 6 years | take 1/4 to 1/2 tablet every 6 to 8 hours; not to exceed 1- 1/2 tablets in 24 hours or as directed by a doctor. |
Motion Sickness Relief Other information
- each tablet contains: calcium 30 mg
- store at room temperature 20o- 25oC (68o - 77o F)
Croscarmellose Sodium, Dicalcium Phosphate, Magnesium Stearate, Microcrystalline Cellulose.
Questions or comments?
Call 1-877-753-3935 Monday- Friday 9AM- 5PM EST
Compare to Active Ingredient in Dramamine® *
Original Formula
Motion Sickness Relief
Dimenhydrinate tablets/ Antiemetic
Fast acting tablets
For children and adults
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
*This product is not manufactured or distributed by Prestige Brands, Inc., owner of the registered trademark Dramamine® Original Formula
Distributed by: FOODHOLD U.S.A LLC
LANDOVER, MD 20785
1-877-846-9949
© 2014 Ahold Licensing, Sarl
Quality guaranteed or your money back
Product Label
Motion Sickness ReliefDimenhydrinate TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||