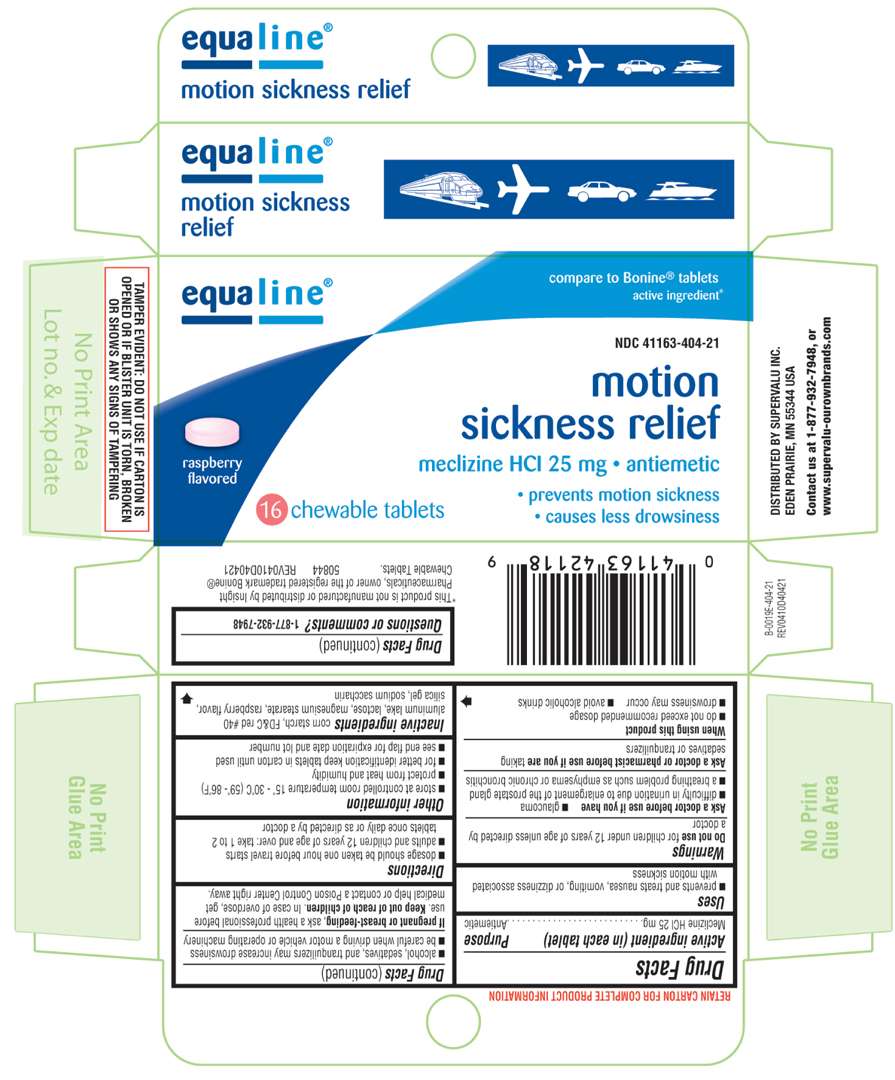
Motion Sickness Relief
Equaine 44-404
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient (in each tablet)
- Purpose
- Use
- Warnings
- Directions
- Motion Sickness Relief Other information
- Inactive ingredients
- Questions or comments?
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredient (in each tablet)
Meclizine HCl 25 mg
Purpose
Antiemetic
Use
- prevents and treats nausea, vomiting, or dizzines associated with motion sickness
Warnings
Do not use
for children under 12 years of age unless directed by a doctor
Ask a doctor before use if you have
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
- a breathing problem such as emphysema or chronic bronchitis
Ask a doctor or pharmacist before use if you are
taking sedatives or tranquilizers
When using this product
- do not exceed recommended dosage
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careul when driving a motor vehicle or operating machinery
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center rigth away.
Directions
- dosage should be taken one hour before travel starts
- adults and children 12 years of age and over: take 1 to 2 tablets once daily or as directed by a doctor
Motion Sickness Relief Other information
- store at controlled room temperature 15º-30ºC (59º-86ºF)
- protect from heat and humidity
- for better identification keep talbets in carton until used
- see end flap for expiration date and lot number
Inactive ingredients
corn starch, FD&C red #40 aluminum lake, lactose, magnesium stearate, rasberry flavor, silica gel, sodium saccharin
Questions or comments?
1-877-932-7948
Principal Display Panel
equaline®
compare to Bonine® tablets active ingredients*
NDC 41163-404-21
motion
sickness relief
meclizine HCl 25 mg • antiemetic
• prevents motion sickness
• causes less drowsiness
16 chewable tablets
*This product is not manufactured or distributed by Insight Pharmaceuticals, owner of the registered trademark Bonine® Chewable Tablets.
50844 REV0410D40421
DISTRIBUTED BY SUPERVALU INC.
EDEN PRARIE, MN 55344 USA
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
Motion Sickness ReliefMeclizine HCl TABLET, CHEWABLE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||