Mucus Relief DM
ASSURED / DOLLAR TREE (Greenbrier International, Inc.)
P and L Development of New York Corporation
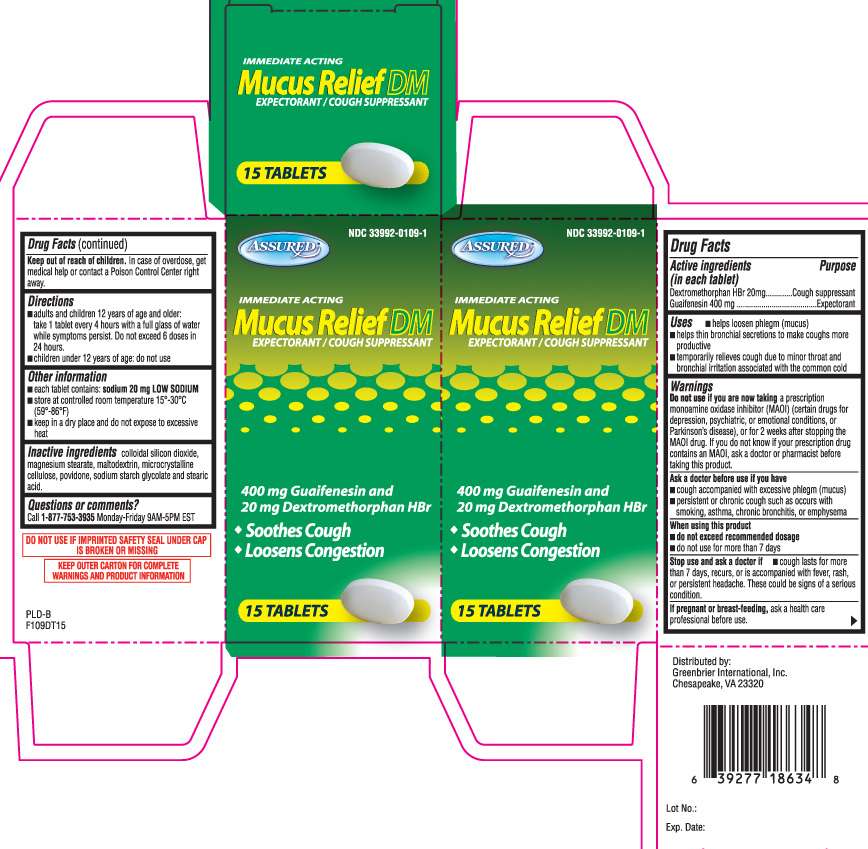
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredients (in each tablet)
- Purpose
- Mucus Relief DM Uses
- Warnings
- Directions
- Mucus Relief DM Other information
- Inactive ingredients
- Questions or comments?
- Principal Display Panel
- Product Label
FULL PRESCRIBING INFORMATION
Active ingredients (in each tablet)
Dextromethorphan HBr 20 mg
Guaifenesin 400 mg
Purpose
Cough suppressant
Expectorant
Mucus Relief DM Uses
- helps loosen phlegm (mucus)
- helps thin bronchial secretions to make coughs more productive
- temporarily relieves cough due to minor throat and bronchial irritation associated with the common cold
Warnings
Do not use if you are now taking
a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- cough accompanied with excessive phlegm (mucus)
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
When using this product
- do not exceed recommended dosage
- do not use for more than 7 days
Stop use and ask a doctor if
- cough lasts for more than 7 days, recurs, or is accompanied with fever, rash, or persistent headache. These could be signs of a serious condition.
If pregnant or breast-feeding,
ask a health care professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 12 years of age and older: take 1 tablet every 4 hours with a full glass of water while symptoms persist. Do not exceed 6 doses in 24 hours.
- children under 12 years of age: do not use
Mucus Relief DM Other information
- each tablet contains: sodium 20 mg LOW SODIUM
- store at controlled room temperature 15°-30°C (59°-86°F)
- keep in a dry place and do not expose to excessive heat
Inactive ingredients
colloidal silicon dioxide, magnesium stearate, maltodextrin, microcrystalline cellulose, povidone, sodium starch glycolate and stearic acid
Questions or comments?
Call 1-877-753-3935 Monday-Friday 9AM-5PM EST
Principal Display Panel
IMMEDIATE ACTING
Mucus Relief DM
EXPECTORANT / COUGH SUPPRESSANT
400 mg Guaifenesin and 20 mg Dextromethorphan HBr
- Soothes Cough
- Loosens Congestion
TABLETS
DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION
Distributed by:
Greenbrier International, Inc.
Chesapeake, VA 23320
Product Label
Mucus Relief DMDextromethorphan Hydrobromide and Guaifenesin TABLET, COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||