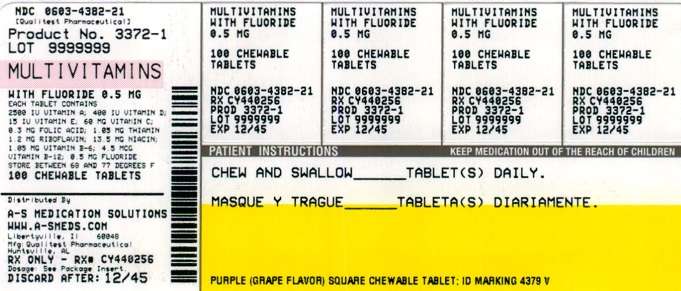
Multi Vitamin with Fluoride
FULL PRESCRIBING INFORMATION: CONTENTS*
- CLINICAL PHARMACOLOGY
- MULTI VITAMIN WITH FLUORIDE INDICATIONS AND USAGE
- WARNING
- PRECAUTIONS
- MULTI VITAMIN WITH FLUORIDE ADVERSE REACTIONS
- MULTI VITAMIN WITH FLUORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
Active ingredient
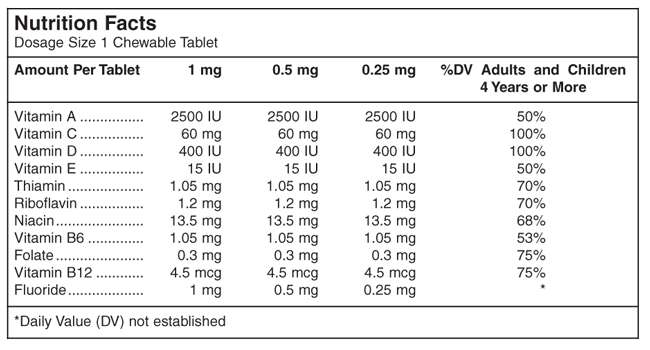
Active ingredient for caries prophylaxis: Fluoride as sodium fluoride.
Other Ingredients:
Artificial grape flavor, ascorbic acid, cholecalciferol, compressible sugar, D&C Red #7 calcium lake, FD&C Blue #1 aluminum lake, folic acid, magnesium stearate, microcrystalline cellulose, niacinamide, polyethylene glycol, pyridoxine, riboflavin, sodium ascorbate, stearic acid, thiamine, vitamin A acetate, vitamin B12 and vitamin E acetate.
CLINICAL PHARMACOLOGY
It is well established that fluoridation of the water supply (1 ppm fluoride) during the period of tooth development leads to a significant decrease in the incidence of dental caries.
Multivitamin with Fluoride Chewable Tablets provide sodium fluoride and ten essential vitamins in a chewable tablet. Because the tablets are chewable, they provide a topical as well as systemic source of fluoride.
Hydroxyapatite is the principal crystal for all calcified tissue in the human body. The fluoride ion reacts with the hydroxyapatite in the tooth as it is formed to produce the more caries-resistant crystal, fluorapatite. The reaction may be expressed by the equation:

Three stages of fluoride deposition in tooth enamel can be distinguished:
- Small amounts (reflecting the low levels of fluoride in tissue fluids) are incorporated into the enamel crystals while they are being formed.
- After enamel has been laid down, fluoride deposition continues in the surface enamel. Diffusion of fluoride from the surface inward is apparently restricted.
- After eruption, the surface enamel acquires fluoride from the water, food, supplementary fluoride and smaller amounts from saliva.
MULTI VITAMIN WITH FLUORIDE INDICATIONS AND USAGE
Supplementation of the diet with ten essential vitamins.
Supplementation of the diet with fluoride for caries prophylaxis.
Multivitamin with 1 mg Fluoride Chewable Tablets provide fluoride in tablet form for children 6-16 years of age in areas where the water fluoride level is less than 0.3 ppm.
Multivitamin with 0.5 mg Fluoride Chewable Tablets provide fluoride in tablet form for children 4-6 years of age where the water fluoride level is less than 0.3 ppm, and for children 6 years of age and above where the drinking water contains 0.3 through 0.6 ppm of fluoride.
Multivitamin with 0.25 mg Fluoride Chewable Tablets provide fluoride in tablet form for children 4-6 years of age where the drinking water contains 0.3 through 0.6 ppm of fluoride.
Multivitamin with Fluoride Chewable Tablets supply significant amounts of Vitamins A, C, D, E, thiamin, riboflavin, niacin, vitamin B6, vitamin B12, and folate to supplement the diet, and to help assure that nutritional deficiencies of these vitamins will not develop. Thus, in a single easy-to-use preparation, children obtain ten essential vitamins and the important mineral, fluoride.
The American Academy of Pediatrics recommends that children up to age 16, in areas where drinking water contains less than optimal levels of fluoride, receive daily fluoride supplementation.
Children using Multivitamin with Fluoride Chewable Tablets regularly should receive semiannual dental examinations. The regular brushing of teeth and attention to good oral hygiene practices are also essential.
WARNING
As in the case of all medications, keep out of the reach of children.
Should be chewed. This product, as all chewable tablets, is not recommended for children under age 4 due to risk of choking.
PRECAUTIONS
The suggested dose of Multivitamin with Fluoride Chewable Tablets should not be exceeded, since dental fluorosis may result from continued ingestion of large amounts of fluoride.
Before prescribing Multivitamin with Fluoride Chewable Tablets:
- Determine the fluoride content of the drinking water from all major sources.
- Make sure the child is not receiving significant amounts of fluoride from other sources such as medications and swallowed toothpaste.
- Periodically check to make sure that the child does not develop significant dental fluorosis.
Do not eat or drink dairy products within one hour of medication administration.
MULTI VITAMIN WITH FLUORIDE ADVERSE REACTIONS
Allergic rash and other idiosyncrasies have been rarely reported.
MULTI VITAMIN WITH FLUORIDE DOSAGE AND ADMINISTRATION
One tablet daily or as prescribed.
HOW SUPPLIED
Multi-vitamin with Fluoride Chewable Tablets Grape are available as follows:
- 1 mg: Bottles of 100: NDC 0603-4383-21
Bottles of 500: NDC 0603-4383-28 - 0.5 mg: Bottles of 100: NDC 0603-4382-21
Bottles of 500: NDC 0603-4382-28 - 0.25 mg: Bottles of 100: NDC 0603-4381-21
Bottles of 500: NDC 0603-4381-28
Manufactured for:
QUALITEST PHARMACEUTICALS
130 Vintage Drive
Huntsville, AL 35811
8183001
R10/12-R3
PRINCIPAL DISPLAY PANEL
NDC 54569-3372-1
Relabeled by:
A-S Medication Solutions
Libertyville, IL 60048
Multi Vitamin with FluorideMulti Vitamin with Fluoride TABLET, CHEWABLE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||