Mustela
Expanscience Laboratoires d/b/a Mustela
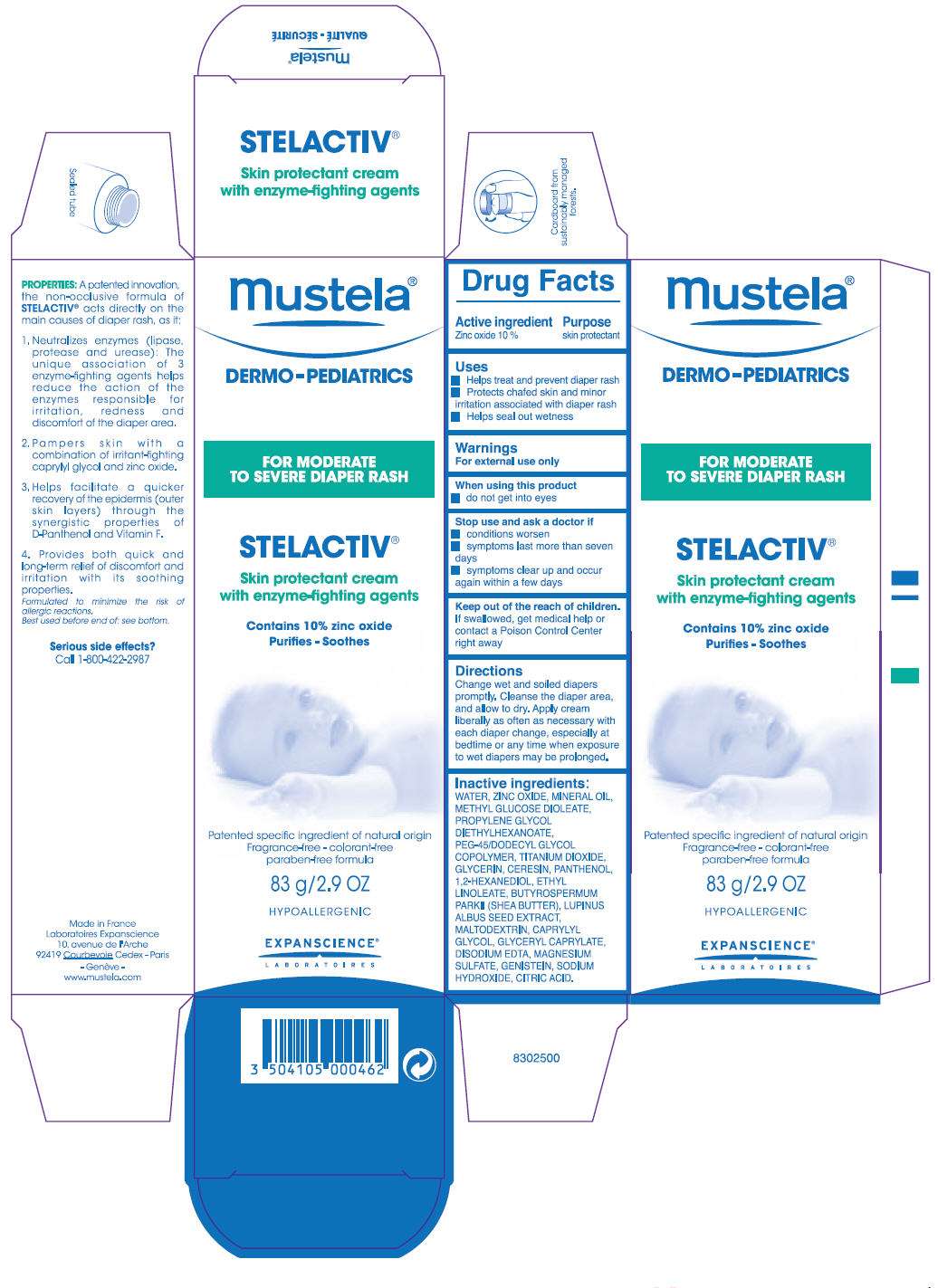
mustela DERMO-PEDIATRICS STELACTIV
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Mustela Uses
- Warnings
- Directions
- Inactive ingredients
- Serious side effects?
- PRINCIPAL DISPLAY PANEL - 83 g Tube Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Zinc oxide 10 %
Purpose
skin protectant
Mustela Uses
- Helps treat and prevent diaper rash
- Protects chafed skin and minor irritation associated with diaper rash
- Helps seal out wetness
Warnings
For external use only
When using this product
- do not get into eyes
Stop use and ask a doctor if
- conditions worsen
- symptoms last more than seven days
- symptoms clear up and occur again within a few days
Keep out of the reach of children. If swallowed, get medical help or contact a Poison Control Center right away
Directions
Change wet and soiled diapers promptly. Cleanse the diaper area, and allow to dry. Apply cream liberally as often as necessary with each diaper change, especially at bedtime or any time when exposure to wet diapers may be prolonged.
Inactive ingredients
WATER, ZINC OXIDE, MINERAL OIL, METHYL GLUCOSE DIOLEATE, PROPYLENE GLYCOL DIETHYLHEXANOATE, PEG-45/DODECYL GLYCOL COPOLYMER, TITANIUM DIOXIDE, GLYCERIN, CERESIN, PANTHENOL, 1,2-HEXANEDIOL, ETHYL LINOLEATE, BUTYROSPERMUM PARKII (SHEA BUTTER), LUPINUS ALBUS SEED EXTRACT, MALTODEXTRIN, CAPRYLYL GLYCOL, GLYCERYL CAPRYLATE, DISODIUM EDTA, MAGNESIUM SULFATE, GENISTEIN, SODIUM HYDROXIDE, CITRIC ACID.
Serious side effects?
Call 1-800-422-2987
www.mustela.com
PRINCIPAL DISPLAY PANEL - 83 g Tube Carton
mustela ®
DERMO-PEDIATRICS
FOR MODERATE
TO SEVERE DIAPER RASH
STELACTIV ®
Skin protectant cream
with enzyme-fighting agents
Contains 10% zinc oxide
Purifies - Soothes
Patented specific ingredient of natural origin
Fragrance-free - colorant-free
paraben-free formula
83 g/2.9 OZ
HYPOALLERGENIC
EXPANSCIENCE
®
LABORATOIRES
MustelaZinc Oxide LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||