Myambutol
FULL PRESCRIBING INFORMATION: CONTENTS*
- MYAMBUTOL DESCRIPTION
- CLINICAL PHARMACOLOGY
- ANIMAL PHARMACOLOGY
- MYAMBUTOL INDICATIONS AND USAGE
- MYAMBUTOL CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- MYAMBUTOL ADVERSE REACTIONS
- MYAMBUTOL DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
MYAMBUTOL DESCRIPTION
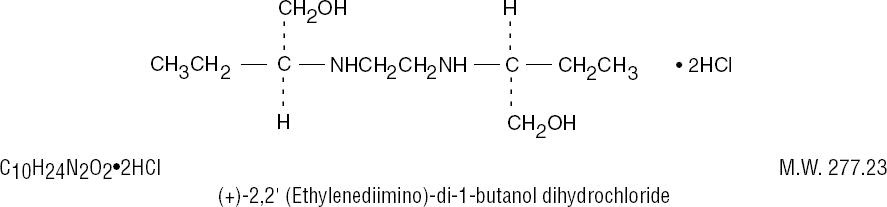
MYAMBUTOL ethambutol hydrochloride is an oral chemotherapeutic agent which is specifically effective against actively growing microorganisms of the genus Mycobacterium, including M. tuberculosis. The structural formula is:
MYAMBUTOL 100 and 400 mg tablets contain the following inactive ingredients: Gelatin, Hydroxypropyl Methylcellulose, Magnesium Stearate, Sodium Lauryl Sulfate, Sorbitol, Stearic Acid, Sucrose, Titanium Dioxide and other ingredients.
CLINICAL PHARMACOLOGY
ANIMAL PHARMACOLOGY
Toxicological studies in dogs on high prolonged doses produced evidence of myocardial damage and failure, and depigmentation of the tapetum lucidum of the eyes, the significance of which is not known. Degenerative changes in the central nervous system, apparently not dose-related, have also been noted in dogs receiving ethambutol hydrochloride over a prolonged period. In the rhesus monkey, neurological signs appeared after treatment with high doses given daily over a period of several months. These were correlated with specific serum levels of ethambutol and with definite neuroanatomical changes in the central nervous system. Focal interstitial carditis was also noted in monkeys which received ethambutol hydrochloride in high doses for a prolonged period.
MYAMBUTOL INDICATIONS AND USAGE
- MYAMBUTOL plus isoniazid
- MYAMBUTOL plus isoniazid plus streptomycin.
MYAMBUTOL CONTRAINDICATIONS
MYAMBUTOL is contraindicated in patients who are known to be hypersensitive to this drug. It is also contraindicated in patients with known optic neuritis unless clinical judgment determines that it may be used. MYAMBUTOL is contraindicated in patients who are unable to appreciate and report visual side effects or changes in vision (e.g., young children, unconscious patients).
WARNINGS
MYAMBUTOL may produce decreases in visual acuity which appear to be due to optic neuritis. This effect may be related to dose and duration of treatment. This effect is generally reversible when administration of the drug is discontinued promptly. However, irreversible blindness has been reported. (See PRECAUTIONS and ADVERSE REACTIONS ).
Liver toxicities including fatalities have been reported (See ADVERSE REACTIONS ). Baseline and periodic assessment of hepatic function should be performed.
PRECAUTIONS
ADVERSE REACTIONS
As with any potent drug, baseline and periodic assessment of organ system functions, including renal, hepatic, and hematopoietic, should be performed.
The results of a study of coadministration of MYAMBUTOL (50mg/kg) with an aluminum hydroxide containing antacid to 13 patients with tuberculosis showed a reduction of mean serum concentrations and urinary excretion of ethambutol of approximately 20% and 13%, respectively, suggesting that the oral absorption of ethambutol may be reduced by these antacid products. It is recommended to avoid concurrent administration of ethambutol with aluminum hydroxide containing antacids for at least 4 hours following ethambutol administration.
MYAMBUTOL is excreted into breast milk. The use of MYAMBUTOL should be considered only if the expected benefit to the mother outweighs the potential risk to the infant.
MYAMBUTOL (ethambutol hydrochloride) is not recommended for use in pediatric patients under thirteen years of age since safe conditions for use have not been established.
There are limited data on the use of MYAMBUTOL in the elderly. One study of 101 patients, 65 years and older, on multiple drug antituberculosis regimens included 94 patients on MYAMBUTOL. No differences in safety or tolerability were observed in these patients compared with that reported in adults in general. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
MYAMBUTOL ADVERSE REACTIONS
| Initial Snellen Reading |
Reading Indicating Significant Decrease |
Significant Number of Lines |
Decrease Number of Points |
| 20/13 | 20/25 | 3 | 12 |
| 20/15 | 20/25 | 2 | 10 |
| 20/20 | 20/30 | 2 | 10 |
| 20/25 | 20/40 | 2 | 15 |
| 20/30 | 20/50 | 2 | 20 |
| 20/40 | 20/70 | 2 | 30 |
| 20/50 | 20/70 | 1 | 20 |
WARNINGS.
MYAMBUTOL DOSAGE AND ADMINISTRATION
Initial Treatment:
In patients who have not received previous antituberculous therapy, administer MYAMBUTOL 15 mg/kg (7 mg/ lb) of body weight, as a single oral dose once every 24 hours. In the more recent studies, isoniazid has been administered concurrently in a single, daily, oral dose.
| 15 mg/kg (7 mg/lb) Schedule | |||
| Weight Range | Daily Dose | ||
| Pounds | Kilograms | In mg | |
| Under 85 lbs. | Under 37 kg | 500 | |
| 85 - 94.5 | 37 – 43 | 600 | |
| 95 - 109.5 | 43 – 50 | 700 | |
| 110 - 124.5 | 50 – 57 | 800 | |
| 125 - 139.5 | 57 – 64 | 900 | |
| 140 - 154.5 | 64 – 71 | 1000 | |
| 155 - 169.5 | 71 – 79 | 1100 | |
| 170 - 184.5 | 79 – 84 | 1200 | |
| 185 - 199.5 | 84 – 90 | 1300 | |
| 200 - 214.5 | 90 – 97 | 1400 | |
| 215 and Over | Over 97 | 1500 | |
| 25 mg/kg (11 mg/lb) Schedule | |||
| Under 85 lbs. | Under 38 kg | 900 | |
| 85 - 92.5 | 38 - 42 | 1000 | |
| 93 - 101.5 | 42 - 45.5 | 1100 | |
| 102 - 109.5 | 45.5 – 50 | 1200 | |
| 110 - 118.5 | 50 – 54 | .1300 | |
| 119 - 128.5 | 54 – 58 | 1400 | |
| 129 - 136.5 | 58 – 62 | 1500 | |
| 137 - 146.5 | 62 – 67 | 1600 | |
| 147 - 155.5 | 67 – 71 | 1700 | |
| 156 - 164.5 | 71 – 75 | 1800 | |
| 165 - 173.5 | 75 – 79 | 1900 | |
| 174 - 182.5 | 79 – 83 | 2000 | |
| 183 - 191.5 | 83 – 87 | 2100 | |
| 192 - 199.5 | 87 – 91 | 2200 | |
| 200 - 209.5 | 91 – 95 | 2300 | |
| 210 - 218.5 | 95 – 99 | 2400 | |
| 219 and Over | Over 99 | 2500 |
HOW SUPPLIED
| Bottles of 60 |
NDC 54868-2876-1 |
PRINCIPAL DISPLAY PANEL
MYAMBUTOL® (Ethambutol Hydrochloride) Tablets USP
400 mg

MyambutolEthambutol hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||