Myoview
Medi-Physics Inc. dba GE Healthcare
MYOVIEW™ 30mL
FULL PRESCRIBING INFORMATION: CONTENTS*
- MYOVIEW DESCRIPTION
- CLINICAL PHARMACOLOGY
- MYOVIEW INDICATIONS AND USAGE
- MYOVIEW CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- MYOVIEW ADVERSE REACTIONS
- MYOVIEW DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL - Kit Label
FULL PRESCRIBING INFORMATION
30 mL
Kit for the Preparation of Technetium Tc99m
Tetrofosmin for Injection
Diagnostic
Radiopharmaceutical
For intravenous use only
MYOVIEW DESCRIPTION
The MYOVIEW 30 mL kit is supplied as a pack of five multi-dose vials for use in the preparation of a technetium Tc99m tetrofosmin intravenous injection to be used for the scintigraphic delineation of regions of reversible myocardial ischemia in the presence or absence of infarcted myocardium and for the evaluation of ventricular function. Each vial contains a predispensed, sterile, non-pyrogenic, lyophilized mixture of 1.38 mg tetrofosmin [6,9-bis(2-ethoxyethyl)-3,12-dioxa-6,9-diphosphatetradecane], 0.09 mg stannous chloride dihydrate (minimum stannous tin 0.015 mg; total stannous and stannic tin 0.0522 mg), 1.92 mg disodium sulphosalicylate, 3 mg sodium D-gluconate, 11 mg sodium hydrogen carbonate, and 3 mg ascorbic acid. The lyophilized powder is sealed under a nitrogen atmosphere with a rubber closure. The product contains no antimicrobial preservative.
The structural formula of tetrofosmin is:
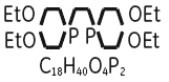
When sterile, pyrogen-free sodium pertechnetate Tc99m in isotonic saline is added to the vial, a Tc99m complex of tetrofosmin is formed.
Administration is by intravenous injection for diagnostic use.
Physical Characteristics
Technetium Tc99m decays by isomeric transition with a physical half-life of 6.03 hours
| Radiation | Mean %/disintegration | Mean energy (keV) |
|---|---|---|
| Gamma 2 | 87.87 | 140.5 |
External Radiation
The specific gamma ray constant for technetium Tc99m is 206 microCoulomb. kg-1/37 MBq-hr (0.8 R/mCi-hr) at 1 cm. The first half-value thickness of lead (Pb) for technetium Tc99m is 0.2 mm.
A range of values for the relative attenuation of the radiation emitted by this radionuclide that results from the interposition of various thicknesses of Pb is shown in Table 2. For example, the use of a 2.7 mm thickness of Pb will decrease the external radiation exposure by a factor of 1000.
| Shield thickness (Pb) mm | Coefficient of attenuation |
|---|---|
| 0.2 | 0.5 |
| 0.95 | 10-1 |
| 1.8 | 10-2 |
| 2.7 | 10-3 |
| 3.6 | 10-4 |
| 4.5 | 10-5 |
To correct for physical decay of this radionuclide, the fractions that remain at selected intervals relative to the time of calibration are shown in Table 3.
| Hours | Fraction Remaining | Hours | Fraction Remaining |
|---|---|---|---|
| 0 |
1.000 | 7 | 0.447 |
| 1 | 0.891 | 8 | 0.399 |
| 2 | 0.795 | 9 | 0.355 |
| 3 | 0.708 | 10 | 0.317 |
| 4 | 0.631 | 11 | 0.282 |
| 5 | 0.563 | 12 | 0.252 |
| 6 | 0.502 | 24 | 0.063 |
CLINICAL PHARMACOLOGY
General
When technetium Tc99m pertechnetate is added to tetrofosmin in the presence of stannous reductant, a lipophilic, cationic technetium Tc99m complex is formed, Tc99m tetrofosmin. This complex is the active ingredient in the reconstituted drug product, on whose biodistribution and pharmacokinetic properties the indications for use depend.
Pharmacokinetics
Studies in normal volunteers have demonstrated rapid myocardial uptake of Tc99m tetrofosmin, and rapid blood, liver and lung clearances. Uptake in the myocardium reaches a maximum of about 1.2% of the injected dose (i.d.) at 5 minutes and approximately 1% of the i.d. at 2 hours, respectively. Background activities in the blood, liver and lung were less than 5% of the administered activity in whole blood at 10 minutes post-injection, less than 4.5% i.d., after 60 minutes, and less than 2% i.d. after 30 minutes. Approximately 66% of the injected activity is excreted within 48 hours post-injection, with approximately 40% excreted in the urine and 26% in the feces.
The kinetics, elimination and protein binding of Tc99m tetrofosmin have not been determined.
Pharmacodynamics
The pharmacodynamic cellular uptake of tetrofosmin in humans has not been established. In humans the recommended imaging time is 15 minutes at stress and 30 minutes at rest.
Metabolism
The metabolic profile of tetrofosmin has not been established.
Drug-Drug Interactions
Specific drug-drug interactions have not been studied.
Clinical Studies
1. Exercise Stress Studies
A total of 252 subjects with ischemic heart disease or atypical chest pain who had a reason for exercise stress imaging were studied in two open-label, multi-center, clinical studies of MYOVIEW (study a and study b). Of these 252 subjects there were 212 (84%) males and 40 (16%) females with a mean age of 60.5 years (range 33.7 to 82.4 years). At peak exercise, maximum heart rate achieved and peak systolic blood pressure were comparable after MYOVIEW and thallium-201 exercise studies.
All subjects had exercise and rest planar imaging with MYOVIEW and thallium-201; 191 (76%) subjects also had single photon emission computed tomography (SPECT) imaging. The MYOVIEW and thallium-201 images were separated by a mean of 5.1 days (1-14 days before or 2-14 days after MYOVIEW). For MYOVIEW imaging, each subject received 185-296 MBq (5-8 mCi) Tc99m tetrofosmin at peak exercise and 555-888 MBq (15-24 mCi) Tc99m tetrofosmin at rest approximately 4 hours later. For thallium-201 imaging, subjects received thallium-201 55.5-74 MBq (1.5-2.0 mCi) at peak exercise.
The images were evaluated for the quality of the image (excellent, good or poor) and the diagnosis (with scores of 0 = normal, 1 = ischemia, 2 = infarct, 3 = mixed infarct and ischemia). The primary outcome variable was the percentage of correct diagnoses in comparison to the final clinical diagnosis. All planar images were blindly read; SPECT images were evaluated by the unblinded investigator. A subset of 181/252 (72%) subjects had coronary angiography comparisons to the planar images of MYOVIEW or thallium-201.
In comparison to the clinical diagnosis, results of MYOVIEW and thallium-201 imaging were comparable within the 95% confidence intervals. The results for each blinded reader are noted in Table 4.
| Total percentage correctly diagnosed | thallium-201 | MYOVIEW™ | |||
|---|---|---|---|---|---|
| Ischemia | Reader 1 | Reader 2 | Reader 1 | Reader 2 | |
| Study a | 77.7% | 75.0% | 66.3% | 63.6% | |
| Study b | 75.6% | 68.9% | 66.4% | 66.4% | |
| Infarct | |||||
| Study a | 75.9% | 75.0% | 75.9% | 75.0% | |
| Study b | 70.6% | 69.7% | 73.1% | 68.1% | |
2. Pharmacologic Stress Studies
MYOVIEW imaging after pharmacologic stress was evaluated in two studies in subjects with known or suspected coronary artery disease (CAD). Three blinded reads were obtained for 57 subjects (45 male [79%], 12 female [21%]; mean age 60.1 years) all of whom had angiography. A subset of 19 subjects also had thallium-201 SPECT imaging. Subject level analyses were based on the finding of SPECT myocardial perfusion abnormalities in patients with angiographically confirmed disease. Subject level sensitivities for MYOVIEW ranged from 68-83% across readers and studies. Subject level sensitivities for thallium-201 ranged from 79-89% across readers.
3. Ventricular Function Studies
Two open-label, multicenter, identically designed, blinded image read clinical studies were conducted to assess left ventricular function using MYOVIEW ECG gated SPECT (GSPECT) myocardial perfusion imaging. A total of 329 subjects (216 male [65.7%], 113 female [34.3%]); mean age of 60.4 years) with known or suspected heart disease or requiring ventricular function assessments were dosed with MYOVIEW. Of these, 297 were considered evaluable. MYOVIEW was administered at rest and at peak stress using either a one-day or a 2-day dosing protocol.
For both studies, all subjects' stress GSPECT exams were compared to the reference exam of radionuclide ventriculography with Tc99m labeled RBCs (multiple gated acquisition [MUGA]), performed 1 to 5 days after the second MYOVIEW injection. All subjects' GSPECT exams were assessed by 3 independent blinded readers per study. The MUGA exams were evaluated as a truth standard by an independent consensus panel composed of 3 blinded readers. Subject level assessments were based upon discrimination between normal and abnormal values for LVEF (LVEF ≥50% was considered normal) and normal and abnormal wall motion as judged visually. Sensitivity and specificity of LVEF determinations ranged from 81%-88% and 76%-85% respectively (across studies and readers). Sensitivity and specificity of wall motion determinations ranged from 80%-92% and 68%-86% respectively (across studies and readers). In an analysis of LVEF by severity of dysfunction (i.e. mild dysfunction 40%-49%, moderate dysfunction 30%-39%, severe dysfunction of <30%), across subgroups there was individual reader variation in agreement with MUGA. These studies were not designed to determine whether differences between GSPECT and MUGA LVEF values were due to over estimation or under estimation by either of the methods.
MYOVIEW INDICATIONS AND USAGE
MYOVIEW is indicated for scintigraphic imaging of the myocardium following separate administrations under exercise and/or resting conditions. It is useful in the delineation of regions of reversible myocardial ischemia in the presence or absence of infarcted myocardium.
MYOVIEW is also indicated for scintigraphic imaging of the myocardium to identify changes in perfusion induced by pharmacologic stress in patients with known or suspected coronary artery disease.
MYOVIEW is also indicated for the assessment of left ventricular function (left ventricular ejection fraction and wall motion) in patients being evaluated for heart disease.
MYOVIEW CONTRAINDICATIONS
None known.
WARNINGS
In studying patients with known or suspected coronary artery disease, care should be taken to ensure continuous cardiac monitoring and the availability of emergency cardiac treatment.
Pharmacologic induction of cardiovascular stress may be associated with serious adverse events such as myocardial infarction, arrhythmia, hypotension, bronchoconstriction, and cerebrovascular events. Caution should be used when pharmacologic stress is selected as an alternative to exercise; it should be used when indicated and in accordance with the pharmacologic stress agent's labeling.
PRECAUTIONS
General
To minimize radiation dose to the bladder, the patient should be encouraged to void when the examination is completed and as often thereafter as possible. Adequate hydration should be encouraged to permit frequent voiding.
The contents of the MYOVIEW vial are intended only for use in the preparation of MYOVIEW Injection and are NOT to be administered directly to the patient.
As with all injectable drug products, allergic reactions and anaphylaxis may occur.
Sometimes Tc99m labeled myocardial imaging agents may produce planar and SPECT images with different imaging information.
In patients who have a MYOVIEW GSPECT LVEF value or wall motion assessment that is not consistent with the clinical status, additional evaluations with other modalities should be considered as appropriate.
MYOVIEW Injection, like other radioactive drugs, must be handled with care and appropriate safety measures should be used to minimize radiation exposure to clinical personnel. Care should also be taken to minimize radiation exposure to the patient consistent with proper patient management.
Radiopharmaceuticals should be used by or under the control of physicians who are qualified by specific training and experience in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radionuclides.
Drug Interactions
Drug interactions were not noted and were not studied in clinical studies in which MYOVIEW was administered to subjects receiving concomitant medication. Drugs such as beta blockers, calcium blockers and nitrates may influence myocardial function and blood flow. The effects of such drugs on imaging results are not known.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies have not been conducted to evaluate carcinogenic potential or effects on fertility.
Tetrofosmin sulphosalicylate was not mutagenic in vitro in the Ames test, mouse lymphoma, or human lymphocyte tests, nor was it clastogenic in vivo in the mouse micronucleus test.
Pregnancy Category C
Animal reproduction studies have not been conducted with MYOVIEW. It is not known whether MYOVIEW can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Therefore, MYOVIEW should not be administered to a pregnant woman unless the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Technetium Tc99m pertechnetate can be excreted in human milk. Therefore, formula should be substituted for breast milk until the technetium has cleared from the body of the nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
Of 2300 patients in clinical studies of MYOVIEW™ (Kit for the Preparation of Technetium Tc99m Tetrofosmin for Injection), 1053 (46%) patients were 65 or older and 270 (12%) were 75 or older. No overall differences in safety were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
MYOVIEW ADVERSE REACTIONS
Adverse events were evaluated in clinical studies (using an exercise/rest protocol) of 764 adults (511 men and 253 women) with a mean age of 58.7 years (range 29-94 years). The subjects received a mean dose of 7.67 mCi on the first injection and 22.4 mCi on the second injection of MYOVIEW.
Deaths did not occur during the clinical study period of 2 days. Six cardiac deaths occurred 3 days to 6 months after injection and were thought to be related to the underlying disease or cardiac surgery. After MYOVIEW injection, serious episodes of angina occurred in 4 subjects, ventricular tachycardia in 1 subject, and respiratory arrest in 1 subject. The respiratory arrest occurred within minutes of MYOVIEW and pharmacologic stress dosing in a subject with underlying pulmonary disease. The patient was treated and fully recovered. Whether this event was caused by underlying disease or concurrent use of MYOVIEW injection and a pharmacologic stress agent cannot be determined.
Overall cardiac adverse events occurred in less than 1% of subjects after MYOVIEW injection. The following events were noted in less than 1% of subjects:
Cardiovascular: angina, hypertension, Torsades de Pointes.
Gastrointestinal: vomiting, abdominal discomfort.
Hypersensitivity: cutaneous allergy, hypotension, dyspnea.
Special Senses: metallic taste, burning of the mouth, smell alteration.
There was a low incidence (less than 4%) of a transient and clinically insignificant rise in white blood cell counts following administration of the agent.
Adverse events were also evaluated in clinical studies using pharmacologic stress. In four studies of 438 adults (232 men and 205 women; gender was not recorded for one subject) with a mean age of 65.4 years (range 27-97 years; age was not recorded for two subjects) received a single pharmacologic stress agent. The subjects received a mean dose of 7.46-7.79 mCi on the rest/first injection and 22.12 - 33.79 mCi on the stress/second injection.
Among the 438 subjects, 319 experienced an adverse event (73%). Events occurring in ≥1% of the subjects included angina (39%), flushing (36%), dyspnea (28%), headache (14%), abdominal pain (11%), dizziness (7%), palpitations (2%), nausea (2%), hypotension (1%) and pain (1%). Events occurring in <1% include cough, arrhythmia, bronchospasm, ECG abnormalities, hypertension, vomiting and asthenia. Attribution of the above events to MYOVIEW and/or the pharmacologic stress agent cannot be determined due to study design.
In additional ventricular function studies with 1 and 2 day dosing protocols, the overall adverse event profile was similar to previous reports and included one subject who was withdrawn for syncope.
Post-marketing experience
The following are spontaneously reported adverse reactions from post-marketing experience. Because the reports cite reactions reported spontaneously from worldwide post-marketing experience, frequency of reactions and the role of MYOVIEW in their causation cannot be reliably determined. The most common adverse reactions reported included the following: rash, urticaria, abnormal vision, allergic reactions, and fever.
MYOVIEW DOSAGE AND ADMINISTRATION
The recommended dose range for MYOVIEW is 5-33 mCi (185-1221 MBq).
For stress and rest imaging, 2 separate doses of MYOVIEW are used. When rest and stress injections are administered on the same day, the first dose should be 5-12 mCi (185-444 MBq) and followed by the second dose of 15-33 mCi (555-1221 MBq) given approximately 1 to 4 hours later.
Imaging may begin 15 minutes following administration of the agent.
Dose adjustment has not been established in renally or liver impaired, pediatric or geriatric patients.
RADIATION DOSIMETRY
Based on human data, the absorbed radiation doses to an average human adult (70 kg) from intravenous injections of the agent under exercise and resting conditions are listed in Table 5. The values are listed in descending order as rad/mCi and µGy/MBq and assume urinary bladder emptying at 3.5 hours.
| Absorbed radiation dose | ||||
|---|---|---|---|---|
| Exercise | Rest | |||
| Target organ | rad/mCi | µGy/MBq | rad/mCi | µGy/MBq |
| Gall bladder wall | 0.123 | 33.2 | 0.180 | 48.6 |
| Upper large intestine | 0.075 | 20.1 | 0.113 | 30.4 |
| Bladder wall | 0.058 | 15.6 | 0.071 | 19.3 |
| Lower large intestine | 0.057 | 15.3 | 0.082 | 22.2 |
| Small intestine | 0.045 | 12.1 | 0.063 | 17.0 |
| Kidney | 0.039 | 10.4 | 0.046 | 12.5 |
| Salivary glands | 0.030 | 8.04 | 0.043 | 11.6 |
| Ovaries | 0.029 | 7.88 | 0.035 | 9.55 |
| Uterus | 0.027 | 7.34 | 0.031 | 8.36 |
| Bone surface | 0.023 | 6.23 | 0.021 | 5.58 |
| Pancreas | 0.019 | 5.00 | 0.018 | 4.98 |
| Stomach | 0.017 | 4.60 | 0.017 | 4.63 |
| Thyroid | 0.016 | 4.34 | 0.022 | 5.83 |
| Adrenals | 0.016 | 4.32 | 0.015 | 4.11 |
| Heart wall | 0.015 | 4.14 | 0.015 | 3.93 |
| Red marrow | 0.015 | 4.14 | 0.015 | 3.97 |
| Spleen | 0.015 | 4.12 | 0.014 | 3.82 |
| Muscle | 0.013 | 3.52 | 0.012 | 3.32 |
| Testes | 0.013 | 3.41 | 0.011 | 3.05 |
| Liver | 0.012 | 3.22 | 0.015 | 4.15 |
| Thymus | 0.012 | 3.11 | 0.009 | 2.54 |
| Brain | 0.010 | 2.72 | 0.008 | 2.15 |
| Lungs | 0.008 | 2.27 | 0.008 | 2.08 |
| Skin | 0.008 | 2.22 | 0.007 | 1.91 |
| Breasts | 0.008 | 2.22 | 0.007 | 1.83 |
Dose calculations were performed using the standard MIRD method (MIRD Pamphlet No.1 (rev),Society of Nuclear Medicine, 1976). Effective dose equivalents (EDE) were calculated in accordance with ICRP 53 (Ann. ICRP 18 (1-4),1988) and gave values of 8.61 × 10-3 mSV/MBq and 1.12 × 10-2 mSV/MBq after exercise and rest, respectively.
INSTRUCTIONS FOR THE PREPARATION OF MYOVIEW 30 mL INJECTION
USE ASEPTIC TECHNIQUE THROUGHOUT.
The user should wear waterproof gloves and use shielding at all times when handling the reconstituted vial or syringes containing the radioactive agent.
The patient dose should be measured by a suitable radioactivity calibration system immediately prior to administration.
-
1) Place one of the MYOVIEW 30 mL vials in a suitable shielding container and sanitize the rubber septum with an isopropyl alcohol swab. Insert a sterile needle (if using a venting needle) through the rubber septum (see Cautionary note 1). -
2) Using sterile, suitable shielded syringes, inject the required activity of up to 2.4 Ci (89 GBq) technetium Tc99m generator eluate and Sodium Chloride Injection, USP, if needed as diluent into the shielded vial to result in a final Tc99m radioactive concentration of no greater than 80 mCi/mL (2.96 GBq/mL) (see Cautionary notes 2,3 and 4). Remove the venting needle. Mix gently for 10 seconds to ensure complete dissolution of the powder. -
3) Incubate at room temperature for 15 minutes. -
4) Assay the total activity, complete the user radiation label and attach it to the vial. -
5) Visually inspect the reconstituted solution at a safe distance through leaded glass. Do not use if it is not clear or if it contains foreign particulate matter. -
6) Withdrawals for administration should be made aseptically using a shielded, sterile syringe and needle (see Cautionary note 5). -
7) Store the reconstituted MYOVIEW 30 mL vial at 2°-25°C (36°-77°F) and use within 12 hours of preparation. Store withdrawals for injection at 2°-25°C (36°-77°F) and use within the same 12 hour period as the reconstituted MYOVIEW 30 mL vial.
Cautionary notes:
-
1) If a venting needle is not used, remove an adequate volume of gas from the vial head space using the same syringe used for the injection of Sodium Chloride Injection, USP or Tc99m generator eluate to avoid over-pressurizing the vial. -
2) The volume of (diluted) technetium Tc99m generator eluate (plus Sodium Chloride Injection, USP as diluent if needed) added to the vial must be in the range 10-30 mL. -
3) If Sodium Chloride Injection, USP, is added directly to the Myoview vial as a diluent , it must be added immediately prior to the addition of Tc99m generator eluate to the vial. -
4) Sodium Chloride Injection, USP must be used as the diluent if needed. The use of bacteriostatic sodium chloride as a diluent for sodium pertechnetate Tc99m injection may adversely affect the radiochemical purity and hence the biological distribution of the MYOVIEW Injection. -
5) A 21 gauge or finer needle is recommended for patient dose preparation. -
6) The pH of the prepared injection should be in the range 7.5-9.0. -
7) Safety and effectiveness of MYOVIEW Injection were established using investigational material shown to have a radiochemical purity of at least 90% prior to administration to patients in clinical studies. -
8) The contents of the MYOVIEW vial are not radioactive. However, after the sodium pertechnetate Tc99m is added adequate shielding of the final preparation must be maintained. -
9) The technetium Tc99m labeling reaction involved in the preparation of MYOVIEW Injection depends on maintaining tin in the divalent (reduced) state. Any oxidant present in the sodium pertechnetate Tc99m used may adversely affect the quality of the preparation. Sodium pertechnetate Tc99m containing oxidants should not be used for the preparation of the labeled product. -
10) The contents of the MYOVIEW vial are sterile and pyrogen-free. The vial contains no bacteriostatic preservative. It is essential that the user follow the directions carefully and adhere to aseptic procedures during the preparation of the radiopharmaceutical.
Quality Control
An assay of the radiochemical purity of the prepared injection can be performed using the following chromatographic procedure.
Equipment and eluent
-
(1) Varian SA TLC strip (2 cm × 20 cm) – Do not heat activate -
(2) Ascending chromatography tank and cover -
(3) 65:35% v/v mixture of acetone and dichloromethane (prepared freshly) -
(4) 1 ml syringe with 22-25 G needle -
(5) Suitable counting equipment
Method
-
(1) Pour the 65:35% v/v acetone:dichloromethane mixture into the chromatography tank to a depth of 1 cm and cover the tank to allow the solvent vapor to equilibrate. -
(2) Mark a Varian SA TLC strip with a pencil line at 3 cm from the bottom and, using an ink marker pen, at 15 cm from the pencil line. The pencil line indicates the origin where the sample is to be applied and movement of color from the ink line will indicate the position of the solvent front when upward elution should be stopped. -
(3) Cutting positions at 3.75 cm and 12 cm above the origin (Rfs 0.25 and 0.8 respectively) should also be marked in pencil. -
(4) Using a 1 ml syringe and needle, apply a 10 µl sample of the prepared injection at the origin of the strip. Do not allow the spot to dry. Place the strip in the chromatography tank immediately and replace the cover. Ensure that the strip is not adhering to the walls of the tank.
Note: A 10 µl sample will produce a spot with a diameter of approximately 10 mm. Different sample volumes have been shown to give unreliable radiochemical purity values. -
(5) When the solvent reaches the ink line, remove the strip from the tank and allow it to dry. -
(6) Cut the strip into 3 pieces at the marked cutting positions and measure the activity on each using suitable counting equipment. Try to ensure similar counting geometry for each piece and minimize equipment dead time losses. -
(7) Calculate the radiochemical purity from:
Do not use material if the radiochemical purity is less than 90%.% MYOVIEW = Activity of center piece × 100 Total activity of all 3 pieces
Note: Free Tc99m pertechnetate runs to the top piece of the strip. MYOVIEW runs to the center piece of the strip. Reduced hydrolyzed Tc99m and any hydrophilic complex impurities remain at the origin in the bottom piece of the strip.
HOW SUPPLIED
The kit comprises five multi-dose vials containing a sterile, non-pyrogenic, freeze dried mixture of tetrofosmin, stannous chloride dihydrate, disodium sulphosalicylate, sodium D-Gluconate sodium hydrogen carbonate and ascorbic acid, together with appropriate number of radiation labels, and a package insert.
NDC 17156-026-30
STORAGE
Store the kit in a refrigerator at 2°-8°C (36°-46°F). The kit should be protected from light.
Store the reconstituted product at 2°-25°C (36°-77°F), using appropriate radiation shielding. Use reconstituted product within 12 hours of preparation.
This reagent kit is approved for use by persons licensed by the Illinois Emergency Management Agency pursuant to 32 Ill. Code Adm. Section, Section 330.260(a) and 335.4010 or under equivalent licenses of the U.S. Nuclear Regulatory Commission, or an Agreement State.
Manufactured for:
GE Healthcare
Medi-Physics, Inc.
Arlington Heights, IL 60004
by:
GE Healthcare AS
Oslo, Norway
Patent No. 5,045,302
Distributed by:
GE Healthcare
Medi-Physics, Inc.
Arlington Heights, IL 60004
1-800-633-4123 (Toll Free)
MYOVIEW is a trademark of GE Healthcare.
GE and the GE Monogram are trademarks of General Electric Company.
© 2011 General Electric Company – All rights reserved.
43-N196B-OSLO
Revised May 2011
PRINCIPAL DISPLAY PANEL - Kit Label
GE Healthcare
N196A
Rx ONLY
Sterile
Pyrogen-free
Diagnostic
For Intravenous
administration only.
MYOVIEW™
30mL
Kit for the Preparation
of Technetium Tc99m
Tetrofosmin for
Injection
5 multi-dose vial pack
NDC 17156-026-30
Contents of Kit
5 STERILE REACTION VIALS
Each vial contains lyophilized form of:
1.38 mg Tetrofosmin
0.09 mg Stannous chloride dihydrate
1.92 mg Disodium sulphosalicylate
3.0 mg Sodium D-gluconate
11.0 mg Sodium hydrogen carbonate
3.0 mg Ascorbic Acid
5 PRESSURE SENSITIVE LABELS
1 PACKAGE INSERT
Myoview is a trademark of GE Healthcare.
GE and the GE Monogram are trademarks
of General Electric Company.
Manufactured for:
GE Healthcare
Medi-Physics Inc.
Arlington Heights, IL 60004
by: GE Healthcare AS, Oslo, Norway
EXP
LOT

MyoviewTetrofosmin INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||