Eckson Labs, LLC
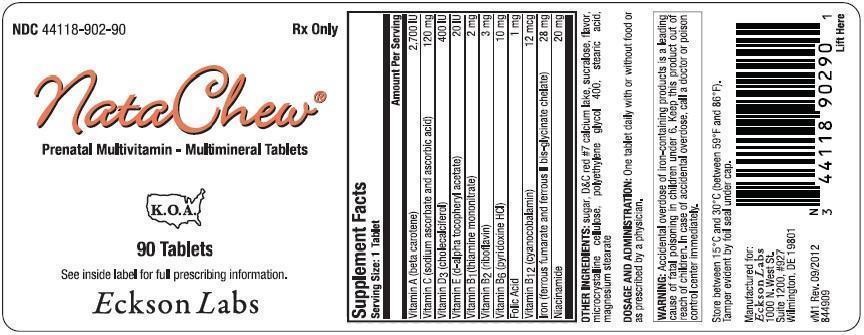
NataChew
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Supplement Facts
Serving Size: 1 Tablet
|
|
|
Amount Per Serving
|
| Vitamin A (beta carotene) |
2,700 IU |
| Vitamin C (sodium ascorbate and ascorbic acid) |
120 mg |
| Vitamin D3 (cholecalciferol) |
400 IU |
| Vitamin E (d-alpha tocopheryl acetate) |
20 IU |
| Vitamin B1 (thiamine mononitrate) |
2 mg |
| Vitamin B2 (riboflavin) |
3 mg |
| Vitamin B6 (pyridoxine HCl) |
10 mg |
| Folic Acid |
1 mg |
| Vitamin B12 (cyanocobalamin) |
12 mcg |
| Iron (ferrous fumarate and ferrous II bis-glycinate chelate) |
28 mg |
| Niacinamide |
20 mg |
OTHER INGREDIENTS: sugar, D&C red #7 calcium lake, sucralose, flavor, microcrystalline cellulose, polyethylene glycol 400, stearic acid, magnesium stearate
INDICATIONS AND USAGE:
®CONTRAINDICATIONS:
WARNINGS AND PRECAUTIONS:
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately.
|
KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN.ADVERSE REACTIONS:
You should call your doctor for medical advice about side effects. To report a serious adverse event, call 1-855-899-4237.DOSAGE AND ADMINISTRATION:
HOW SUPPLIED:
®
NataChew
.BETA.-CAROTENE, ASCORBIC ACID, CHOLECALCIFEROL, .ALPHA.-TOCOPHEROL ACETATE, D-, THIAMINE MONONITRATE, RIBOFLAVIN, PYRIDOXINE HYDROCHLORIDE, FOLIC ACID, CYANOCOBALAMIN, IRON, NIACINAMIDE TABLET, CHEWABLE
Product Information
|
|
Product Type
|
Human prescription drug label |
Item Code (Source)
|
NDC:44118-902 |
|
Route of Administration
|
ORAL |
DEA Schedule
|
|
Product Characteristics
|
|
Color
|
Size
|
Imprint Code
|
Shape
|
|
red |
13 mm |
902 |
ROUND |
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:44118-902-90 |
90 in 1 BOTTLE, PLASTIC |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
|
|
2012-10-15 |
|
|
