Nazal
DRUG FACTS
FULL PRESCRIBING INFORMATION
Active ingredient
Active ingredients
Naphazoline hydrochloride 0.05%
Purpose
Purpose Nasal decongestant
Uses
Uses for the temporary relief of nasal congestion due to the common cold, hay fever, or associated with sinusitis
Warnings
For external use only
Do not use
■for more than 3 days
■in children under 12 years of age because it may cause sedation if swallowed
Ask a doctor before use if you have
■heart disease
■high blood pressure
■thyroid disease ■diabetes
■difficulty in urination due to enlargement of the prostate gland
When using this product
■do not exceed recommended dosage
■if may cause temporary discomfort such as burning, stinging, sneezing, or an increase in nasal discharge
■the use of this container by more than one person may spread infection
■use only as directed
■frequent or prolonged use may cause nasal congestion to recur or worsen
Stop use and ask a doctor if
■symptoms persist
If pregnant or breast-feeding, ask a health professional before use.
Keep our of reach of children. In case of accidental overdose, get medical help or contact a Poison Control Center right away.
Directions
Adults and children 12 years of age and older - 1 or 2 sprays in each nostril not more often than every 6 hours
Children under 12 years of age - do not give unless directed by a doctor
Other information
■container is filled to proper level for best spray action
Inactive ingredients
benzalkonium chloride, citric acid, dibasic potassium phosphate, fragrance, monobasic potassium phosphate, simethicone, sodium chloride, water
nazalPDP.jpg
nazalcart.jpg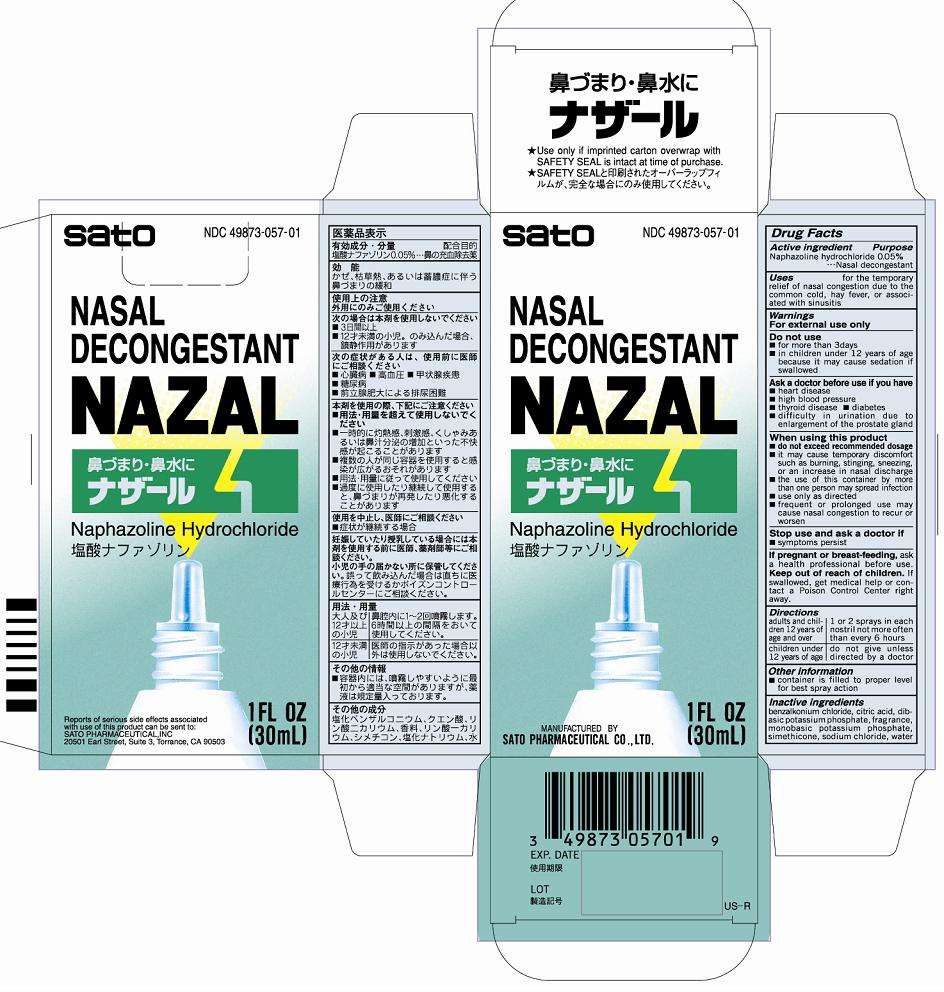
NazalNaphazoline hydrochloride LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||