Nesacaine
General Injectables & Vaccines, Inc
Nesacaine 1% 10mg/mL 30mL Multiple Dose Vial
FULL PRESCRIBING INFORMATION: CONTENTS*
- NESACAINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- INDICATIONS & USAGE
- NESACAINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- NESACAINE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL
FULL PRESCRIBING INFORMATION
NESACAINE DESCRIPTION
NESACAINE - chloroprocaine hydrochloride injection, solution
NESACAINE MPF - chloroprocaine hydrochloride injection, solution
APP Pharmaceuticals, LLC
For Infiltration and Nerve Block
DESCRIPTION
Nesacaine and Nesacaine-MPF Injections are sterile non pyrogenic local anesthetics. The active ingredient in Nesacaine and
Nesacaine-MPF Injections is chloroprocaine HCl (benzoic acid, 4-amino-2-chloro-2-(diethylamino) ethyl ester, monohydrochloride),
which is represented by the following structural formula:
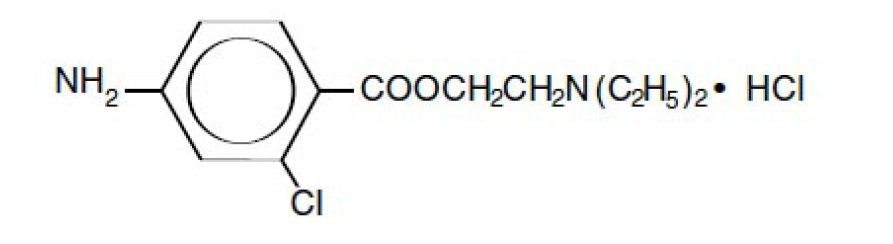
Table 1: Composition of Available Injections
| Product Indentification |
Chloroprocaine HCl |
Sodium Chloride |
Disodium EDTA dihydrate |
Methylparaben |
| Nesacaine 1% |
10 |
6.7 |
0.111 |
1 |
| Nesacaine 2% |
20 |
4.7 |
0.111 |
1 |
| Nesacaine-MPF 2% |
20 |
4.7 |
- |
- |
| Nesacaine-MPF 3% |
30 |
3.3 |
- |
- |
|
|
|
|
|
|
CLINICAL PHARMACOLOGY
Chloroprocaine, like other local anesthetics, blocks the generation and the conduction of nerve impulses, presumably by increasing the
threshold for electrical excitation in the nerve, by slowing the propagation of the nerve impulse and by reducing the rate of rise of the
action potential. In general, the progression of anesthesia is related to the diameter, myelination and conduction velocity of affected
nerve fibers. Clinically, the order of loss of nerve function is as follows: (1) pain, (2) temperature, (3) touch, (4) proprioception, and
(5) skeletal muscle tone.
Systemic absorption of local anesthetics produces effects on the cardiovascular and central nervous systems. At blood concentrations
achieved with normal therapeutic doses, changes in cardiac conduction, excitability, refractoriness, contractility, and peripheral
vascular resistance are minimal. However, toxic blood concentrations depress cardiac conduction and excitability, which may lead to
atrioventricular block and ultimately to cardiac arrest. In addition, with toxic blood concentrations myocardial contractility may be
depressed and peripheral vasodilation may occur, leading to decreased cardiac output and arterial blood pressure.
Following systemic absorption, toxic blood concentrations of local anesthetics can produce central nervous system stimulation,
depression, or both. Apparent central stimulation may be manifested as restlessness, tremors and shivering, which may progress to
convulsions. Depression and coma may occur, possibly progressing ultimately to respiratory arrest.
However, the local anesthetics have a primary depressant effect on the medulla and on higher centers. The depressed stage may occur
without a prior stage of central nervous system stimulation.
PHARMACOKINETICS
The rate of systemic absorption of local anesthetic drugs is dependent upon the total dose and concentration of drug administered,
the route of administration, the vascularity of the administration site, and the presence or absence of epinephrine in the anesthetic
injection. Epinephrine usually reduces the rate of absorption and plasma concentration of local anesthetics and is sometimes added to
local anesthetic injections in order to prolong the duration of action.
INDICATIONS & USAGE
Nesacaine 1% and 2% Injections, in multidose vials with methylparaben as preservative, are indicated for the production of local
anesthesia by infiltration and peripheral nerve block. They are not to be used for lumbar or caudal epidural anesthesia.
Nesacaine-MPF 2% and 3% Injections, in single dose vials without preservative and without EDTA, are indicated for the production
of local anesthesia by infiltration, peripheral and central nerve block, including lumbar and caudal epidural blocks.
Nesacaine and Nesacaine-MPF Injections are not to be used for subarachnoid administration.
NESACAINE CONTRAINDICATIONS
Nesacaine and Nesacaine-MPF Injections are contraindicated in patients hypersensitive (allergic) to drugs of the PABA ester group.
Lumbar and caudal epidural anesthesia should be used with extreme caution in persons with the following conditions: existing
neurological disease, spinal deformities, septicemia, and severe hypertension.
WARNINGS
LOCAL ANESTHETICS SHOULD ONLY BE EMPLOYED BY CLINICIANS WHO ARE WELL VERSED IN DIAGNOSIS AND
MANAGEMENT OF DOSE RELATED TOXICITY AND OTHER ACUTE EMERGENCIES WHICH MIGHT ARISE FROM
THE BLOCK TO BE EMPLOYED, AND THEN ONLY AFTER ENSURING THE IMMEDIATE AVAILABILITY OF OXYGEN,
OTHER RESUSCITATIVE DRUGS, CARDIOPULMONARY RESUSCITATIVE EQUIPMENT, AND THE PERSONNEL
RESOURCES NEEDED FOR PROPER MANAGEMENT OF TOXIC REACTIONS AND RELATED EMERGENCIES (see also
ADVERSE REACTIONS and PRECAUTIONS). DELAY IN PROPER MANAGEMENT OF DOSE RELATED TOXICITY,
UNDERVENTILATION FROM ANY CAUSE AND/OR ALTERED SENSITIVITY MAY LEAD TO THE DEVELOPMENT
OF ACIDOSIS, CARDIAC ARREST AND, POSSIBLY, DEATH. NESACAINE (chloroprocaine HCl Injection, USP) contains
methylparaben and should not be used for lumbar or caudal epidural anesthesia because safety of this antimicrobial preservative has
not been established with regard to intrathecal injection, either intentional or unintentional. NESACAINE-MPF Injection contains no
preservative; discard unused injection remaining in vial after initial use.
Intra-articular infusions of local anesthetics following arthroscopic and other surgical procedures is an unapproved use, and there have
been post-marketing reports of chondrolysis in patients receiving such infusions. The majority of reported cases of chondrolysis have
involved the shoulder joint; cases of gleno-humeral chondrolysis have been described in pediatric and adult patients following intraarticular
infusions of local anesthetics with and without epinephrine for periods of 48 to 72 hours. There is insufficient information to
determine whether shorter infusion periods are not associated with these findings. The time of onset of symptoms, such as joint pain,
stiffness and loss of motion can be variable, but may begin as early as the 2nd month after surgery. Currently, there is no effective
treatment for chondrolysis; patients who experienced chondrolysis have required additional diagnostic and therapeutic procedures and
some required arthroplasty or shoulder replacement.
Vasopressors should not be used in the presence of ergot-type oxytocic drugs, since a severe persistent hypertension may occur.
To avoid intravascular injection, aspiration should be performed before the anesthetic solution is injected. The needle must be
repositioned until no blood return can be elicited. However, the absence of blood in the syringe does not guarantee that intravascular
injection has been avoided.
Mixtures of local anesthetics are sometimes employed to compensate for the slower onset of one drug and the shorter duration of
action of the second drug. Experiments in primates suggest that toxicity is probably additive when mixtures of local anesthetics
are employed, but some experiments in rodents suggest synergism. Caution regarding toxic equivalence should be exercised when
mixtures of local anesthetics are employed.
PRECAUTIONS
General
The safety and effective use of chloroprocaine depend on proper dosage, correct technique, adequate precautions and readiness for
emergencies. Resuscitative equipment, oxygen and other resuscitative drugs should be available for immediate use (see WARNINGS
and ADVERSE REACTIONS). The lowest dosage that results in effective anesthesia should be used to avoid high plasma levels
and serious adverse effects. Injections should be made slowly, with frequent aspirations before and during the injection to avoid
intravascular injection. Syringe aspirations should also be performed before and during each supplemental injection in continuous
(intermittent) catheter techniques. During the administration of epidural anesthesia, it is recommended that a test dose be administered
(3 mL of 3% or 5 mL of 2% Nesacaine-MPF Injection) initially and that the patient be monitored for central nervous system
toxicity and cardiovascular toxicity, as well as for signs of unintended intrathecal administration, before proceeding. When clinical
conditions permit, consideration should be given to employing a chloroprocaine solution that contains epinephrine for the test dose
because circulatory changes characteristic of epinephrine may also serve as a warning sign of unintended intravascular injection. An
intravascular injection is still possible even if aspirations for blood are negative. With the use of continuous catheter techniques,
it is recommended that a fraction of each supplemental dose be administered as a test dose in order to verify proper location of the
catheter.
Injection of repeated doses of local anesthetics may cause significant increases in plasma levels with each repeated dose due to slow
accumulation of the drug or its metabolites. Tolerance to elevated blood levels varies with the physical condition of the patient.
Debilitated, elderly patients, acutely ill patients, and children should be given reduced doses commensurate with their age and physical
status. Local anesthetics should also be used with caution in patients with hypotension or heart block.
Careful and constant monitoring of cardiovascular and respiratory (adequacy of ventilation) vital signs and the patient’s state of
consciousness should be accomplished after each local anesthetic injection. It should be kept in mind at such times that restlessness,
anxiety, tinnitus, dizziness, blurred vision, tremors, depression or drowsiness may be early warning signs of central nervous system
toxicity.
Local anesthetic injections containing a vasoconstrictor should be used cautiously and in carefully circumscribed quantities in areas of
the body supplied by end arteries or having otherwise compromised blood supply. Patients with peripheral vascular disease and those
with hypertensive vascular disease may exhibit exaggerated vasoconstrictor response. Ischemic injury or necrosis may result.
Since ester-type local anesthetics are hydrolyzed by plasma cholinesterase produced by the liver, chloroprocaine should be used
cautiously in patients with hepatic disease.
Local anesthetics should also be used with caution in patients with impaired cardiovascular function since they may be less able to
compensate for functional changes associated with the prolongation of A-V conduction produced by these drugs.
Use in Ophthalmic Surgery: When local anesthetic injections are employed for retrobulbar block, lack of corneal sensation should
not be relied upon to determine whether or not the patient is ready for surgery. This is because complete lack of corneal sensation
usually precedes clinically acceptable external ocular muscle akinesia.
Information for Patients
When appropriate, patients should be informed in advance that they may experience temporary loss of sensation and motor activity,
usually in the lower half of the body, following proper administration of epidural anesthesia.
Clinically Significant Drug Interactions
The administration of local anesthetic solutions containing epinephrine or norepinephrine to patients receiving monoamine oxidase
inhibitors, tricyclic antidepressants or phenothiazines may produce severe, prolonged hypotension or hypertension. Concurrent use of
these agents should generally be avoided. In situations when concurrent therapy is necessary, careful patient monitoring is essential.
Concurrent administration of vasopressor drugs (for the treatment of hypotension related to obstetric blocks) and ergot-type oxytocic
drugs may cause severe, persistent hypertension or cerebrovascular accidents.
The para-aminobenzoic acid metabolite of chloroprocaine inhibits the action of sulfonamides. Therefore, chloroprocaine should not be
used in any condition in which a sulfonamide drug is being employed.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Long-term studies in animals to evaluate carcinogenic potential and reproduction studies to evaluate mutagenesis or impairment of
fertility have not been conducted with chloroprocaine.
Pregnancy: Category C
Animal reproduction studies have not been conducted with chloroprocaine. It is also not known whether chloroprocaine can cause
fetal harm when administered to a pregnant woman or can affect reproduction capacity. Chloroprocaine should be given to a pregnant
woman only if clearly needed. This does not preclude the use of chloroprocaine at term for the production of obstetrical anesthesia.
Labor and Delivery
Local anesthetics rapidly cross the placenta, and when used for epidural, paracervical, pudendal or caudal block anesthesia, can cause
varying degrees of maternal, fetal and neonatal toxicity (see CLINICAL PHARMACOLOGY and PHARMACOKINETICS).
NESACAINE ADVERSE REACTIONS
Systemic: The most commonly encountered acute adverse experiences that demand immediate countermeasures are related to the
central nervous system and the cardiovascular system. These adverse experiences are generally dose related and may result from
OVERDOSAGE
Acute emergencies from local anesthetics are generally related to high plasma levels encountered during therapeutic use of local
anesthetics or to unintended subarachnoid injection of local anesthetic solution (see ADVERSE REACTIONS, WARNINGS and
PRECAUTIONS).
In mice, the intravenous LD50 of chloroprocaine HCl is 97 mg/kg and the subcutaneous LD50 of chloroprocaine HCl is 950 mg/kg.
Management of Local Anesthetic Emergencies: The first consideration is prevention, best accomplished by careful and constant
monitoring of cardiovascular and respiratory vital signs and the patient’s state of consciousness after each local anesthetic injection.
At the first sign of change, oxygen should be administered.
The first step in the management of convulsions, as well as underventilation or apnea due to unintentional subarachnoid injection
of drug solution, consists of immediate attention to the maintenance of a patent airway and assisted or controlled ventilation with
oxygen and a delivery system capable of permitting immediate positive airway pressure by mask. Immediately after the institution of
these ventilatory measures, the adequacy of the circulation should be evaluated, keeping in mind that drugs used to treat convulsions
sometimes depress the circulation when administered intravenously. Should convulsions persist despite adequate respiratory support,
and if the status of the circulation permits, small increments of an ultra-short acting barbiturate (such as thiopental or thiamylal) or a
benzodiazepine (such as diazepam) may be administered intravenously; the clinician should be familiar, prior to the use of anesthetics,
with these anticonvulsant drugs. Supportive treatment of circulatory depression may require administration of intravenous fluids and,
when appropriate, a vasopressor dictated by the clinical situation (such as ephedrine to enhance myocardial contractile force).
If not treated immediately, both convulsions and cardiovascular depression can result in hypoxia, acidosis, bradycardia, arrhythmias
and cardiac arrest. Underventilation or apnea due to unintentional subarachnoid injection of local anesthetic solution may produce
these same signs and also lead to cardiac arrest if ventilatory support is not instituted. If cardiac arrest should occur, standard
cardiopulmonary resuscitative measures should be instituted. Recovery has been reported after prolonged resuscitative efforts.
Endotracheal intubation, employing drugs and techniques familiar to the clinician, may be indicated, after initial administration of
oxygen by mask, if difficulty is encountered in the maintenance of a patent airway or if prolonged ventilatory support (assisted or
controlled) is indicated.
DOSAGE & ADMINISTRATION
Chloroprocaine may be administered as a single injection or continuously through an indwelling catheter. As with all local
anesthetics, the dose administered varies with the anesthetic procedure, the vascularity of the tissues, the depth of anesthesia and
degree of muscle relaxation required, the duration of anesthesia desired, and the physical condition of the patient. The smallest
dose and concentration required to produce the desired result should be used. Dosage should be reduced for children, elderly and
| Anesthetic Procedure |
Solution Concentration% |
Volume (mL) |
Total Dose (mg) |
| Mandibular |
2 |
2-3 |
40-60 |
| Infraorbital |
2 |
0.5-1 |
10-20 |
| Brachial plexus |
2 |
30-40 |
600-800 |
| Digital (without epinephrine) |
1 |
3-4 |
30-40 |
| Pudendal |
2 |
10 each side |
400 |
| Paracervical (see also PRECAUTIONS) |
1 |
3 per each of 4sites |
up to 120 |
HOW SUPPLIED
HOW SUPPLIED
NESACAINE (chloroprocaine HCl Injection, USP) with preservatives is supplied as follows:
| Product No. |
NDC No. |
Strength |
Vial size |
| 470537 |
63323-475-37 |
1% (10 mg/mL) |
30 mL multiple dose vial packaged in trays of 25. |
| 470637 |
63323-476-37 |
2% (20 mg/mL) |
30 mL multiple dose vial packaged in trays of 25. |
| Prduct No. |
NDC No. |
Strength |
Vial size |
| 470727 |
63323-477-27 |
2% (20 mg/mL) |
20 mL single dose vial packaged in trays of 25. |
| 470827 |
63323-478-27 |
3% (30 mg/mL) |
20 mL single dose vial packaged in trays of 25. |
PACKAGE LABEL

NesacaineChloroprocaine Hydrochloride INJECTION, SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||